





Inhalation Article: December 2019
Optimizing the role of automation in variability reduction strategies for delivered dose uniformity (DDU) and aerodynamic particle…
查看文章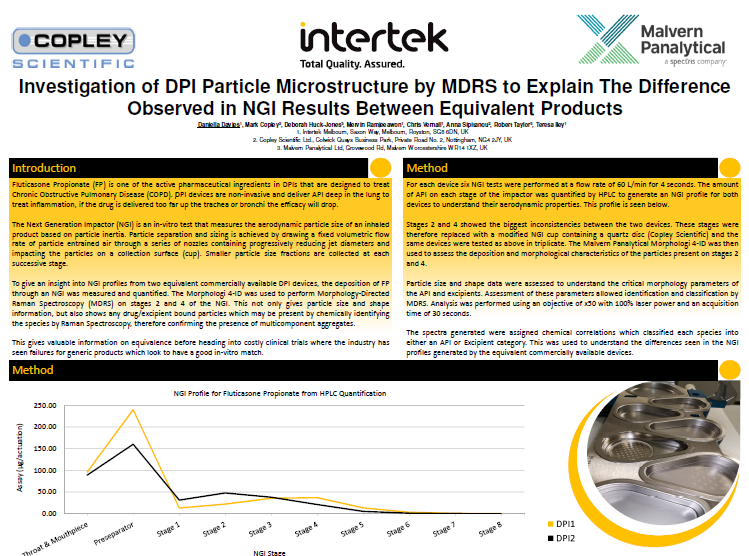

Poster: December 2019
Investigation of DPI particle microstructure by MDRS to explain the difference observed in NGI results between equivalent products
查看文章

Inhalation Article: April 2019
The regulatory landscape for OINDPs – the biggest shake-up in a generation?
查看文章

ONdrugDelivery Article: December 2018
Variability in cascade impaction: sources, impact and strategies for reduction In this article, Mark Copley, Chief Executive Offic…
查看文章

White Paper: October 2018
Analysis technology for inhalation product testing [article in Chinese]
查看文章

Drug Development & Delivery Article: April 2018
Optimizing the Application of In Vitro Test Methods for the Demonstration of Bioequivalence in Orally Inhaled Products
查看文章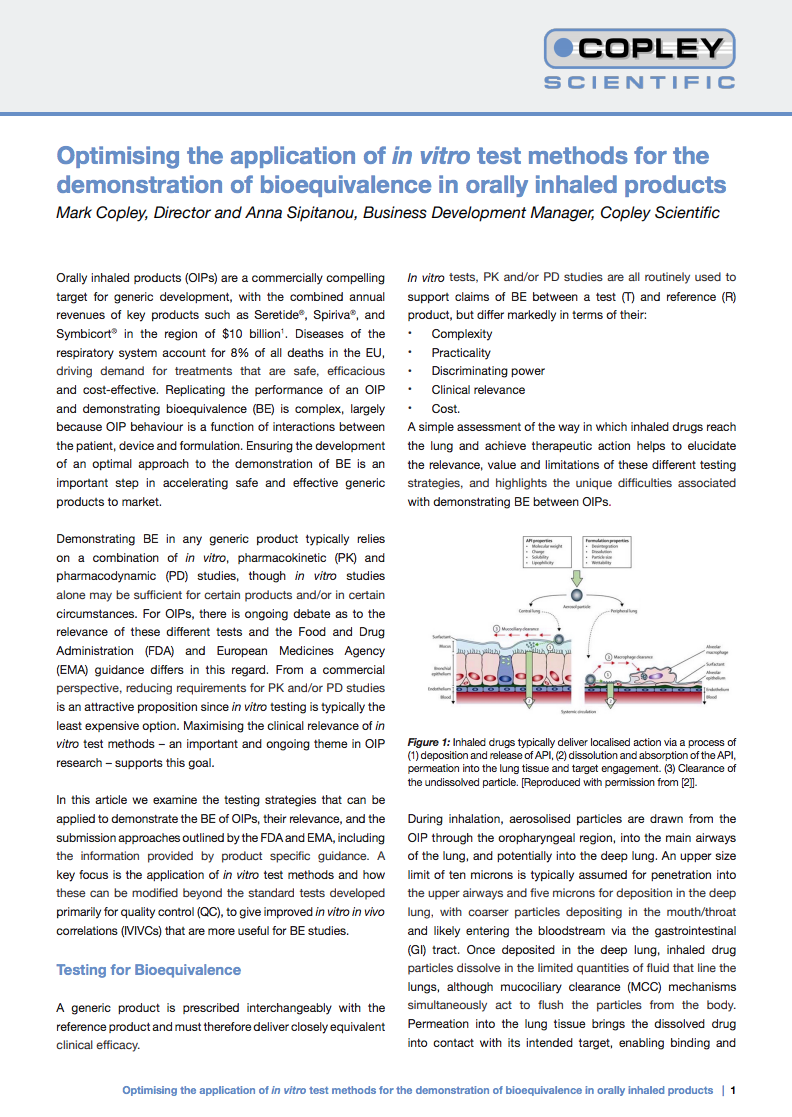

White Paper: February 2018
Optimising the application of in vitro test methods for the demonstration of BE in OIPs
查看文章

Pharmaceutical Technology White Paper 2007 (121kb)
Understanding Cascade Impaction and its Importance for Inhaler Testing
查看文章

PMPS Article (Samedan): February 2018
Demonstrating Bioequivalence of Orally Inhaled Products Safe and cost-effective treatments for respiratory diseases are facing a g…
查看文章


PMPS Article (Samedan): February 2016
Go With the Flow – Testing Inhaled Generics Increasing global requirements for efficacious, inexpensive products to treat re…
查看文章

