





Pharmaceutical Technology Q&A 2014
Exploring newly introduced methods for testing metered dose inhalers with add-on devices The role of add-on devices and how they a…
查看文章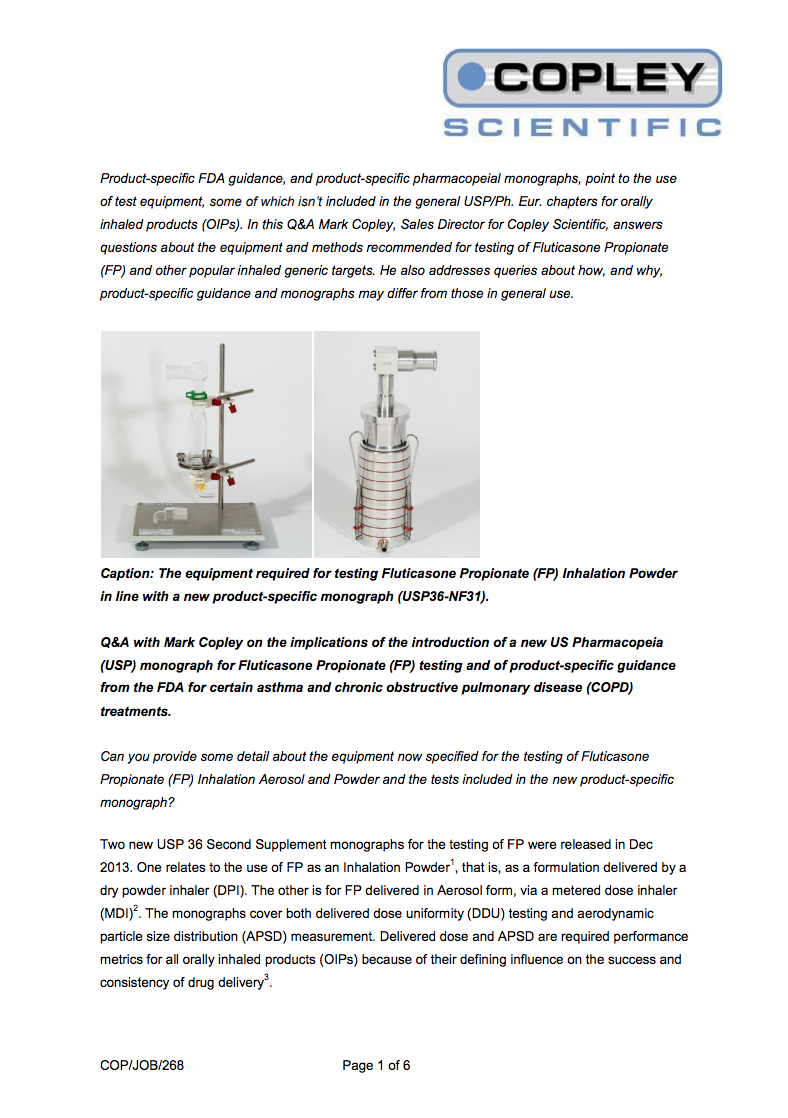

OINDP News Q&A 2014
Implications of the introduction of a new US Pharmacopeia (USP) monograph for Fluticasone Propionate (FP) testing
查看文章

Pharm Bio World Article 2014
Nebulisers – Understanding the Regulatory Framework and Testing Requirements
查看文章





Pharmaceutical Technology Article 2013
Improving Inhaled Product Testing – Methods for Obtaining Better In vitro-In vivo Relationships
查看文章



Pharmaceutical Formulation & Quality Article 2012
Tools of the trade – Cascade impaction and its role in inhaled product development
查看文章

Pharmaceutical Technology Article 2011
Calculating Particle Size Distribution Metrics for Inhaled Product Characterization
查看文章



Pharmaceutical Technology Europe Article 2010
Although there are no regulatory requirements or established pharmacopoeial techniques for the dissolution testing of inhale…
查看文章

