2 March 2017; Nottingham, UK: Copley Scientific, the world’s leading manufacturer of inhaler test equipment, has upgraded its Sample Preparation Unit (SPU) Model 2000 to include a modified induction port fixture with a view to further broadening its appeal. The upgraded SPU 2000 now accommodates a Fluticasone Propionate/Salmeterol Xinafoate (FP/SX) induction port, in addition to the standard Andersen Cascade Impactor (ACI) and Next Generation Impactor (NGI) induction ports, making it a universally applicable labour-saving device for rinsing the induction ports routinely used in cascade impactor testing. Copley Scientific produces equipment to improve in vitro testing of inhaled drug products and has upgraded the SPU 2000 to ensure accurate, reproducible and highly efficient testing across its cascade impactor range.
Conducting well-controlled, highly reproducible cascade impaction tests is critical for obtaining reliable data, for product development and quality control. Cascade impaction is used to measure the aerodynamic particle size distribution of inhaler generated aerosols and is a critically important procedure, but is widely recognised as being both time-consuming and manually intensive. Although not a technically complex task, recovering the active drug sample from the ACI and NGI induction ports, and NGI pre-separator (for which another fixture is available) is highly repetitive and susceptible to human error. Copley’s modified induction port fixture accommodates the new FP/SX induction port and addresses this industry challenge, by semi-automating the process, whilst reducing the potential for error associated with manual drug recovery and freeing up analysts to undertake other tasks.
“Fully automating cascade impactor testing can be challenging, because of its complexity and the need for complex validation and maintenance. We find that many of our customers regard semiautomation as a more practical and cost-effective option. Our latest modification to the SPU 2000 induction port fixture reflects the increasing use of the special FP/SX induction port. This modification enables semi-automated drug recovery from of a wider range of cascade impactor components, making the SPU 2000 an even more cost effective laboratory tool,” – Mark Copley, Sales Director at Copley Scientific.
Inhaled products containing FP and/or SX are currently among the most popular generic targets. This has resulted in the recent release of USP monographs, referencing drug-specific cascade impactor components. Combined with the success of the SPU 2000 and increased customer demand, this is the key driver for the new upgrade. Optimisation of such labour-saving devices contributes to the cost-efficient implementation of vital testing techniques and enhances the quality of results.
To find out more about the new developments go to www.copleyscientific.com
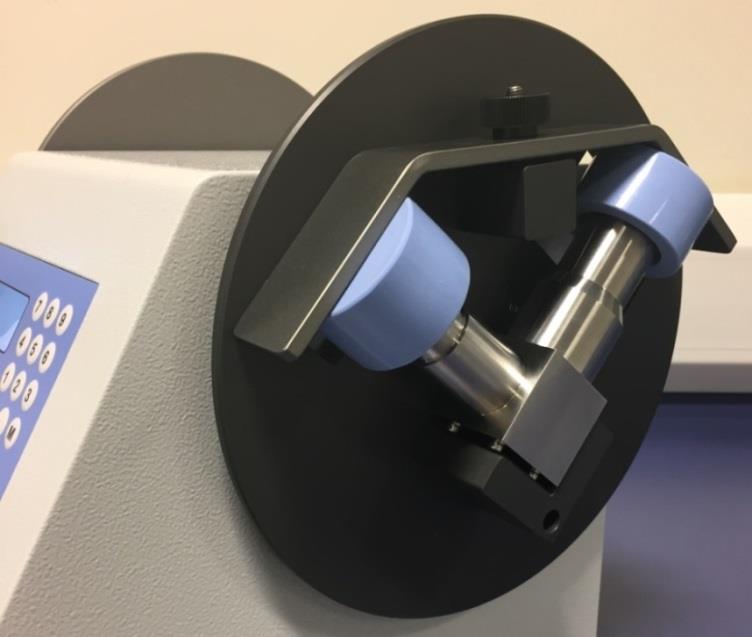
CAPTION: Copley Scientific’s modified SPU 2000 fixture complete with the FP/SX induction port
About Copley Scientific
Copley Scientific is recognised as the world’s leading manufacturer and supplier of inhaler test equipment and is a major provider of testing systems for other pharmaceutical dosage forms. The company is also active in detergent testing.
Copley Scientific’s pharmaceutical product range includes test equipment for delivered dose uniformity and aerodynamic particle size measurement of metered-dose inhalers, dry powder inhalers, nebulizers and nasal sprays; as well as tablets (dissolution, disintegration, friability and hardness) capsules, powders, suppositories, semisolids and transdermals.
Copley Scientific has offices in the UK and Switzerland and works in partnership with aerosol particle science experts MSP Corporation in North America.
Serving the pharmaceutical and detergent industries, Copley Scientific offers an extensive range of equipment for research, development and quality control, as well as full validation and aftersales services. This broad range of products is supplied and supported worldwide through a network of specialist distributors.