Flow Rate-Rise Time Profiles from Model Dry Powder Inhaler (DPI) Testing of Abbreviated Impactor Systems Compared with Their Full Resolution Counterparts Initial Experimental Data
Jolyon Mitchell1, Daryl Roberts2, Henk Versteeg3, Andy Cooper4, Mark Copley5, Roland Greguletz6
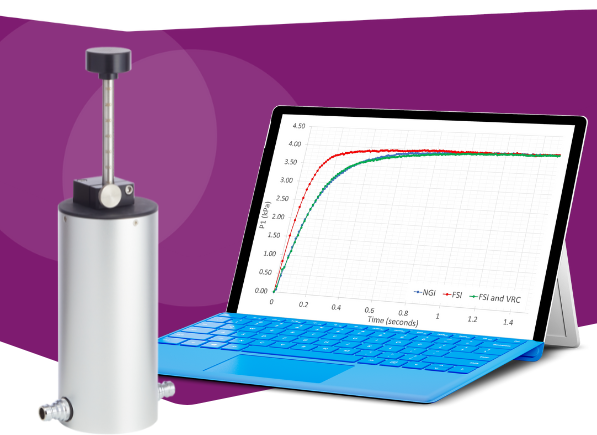
A previous EPAG cross-industry study investigated flow rate-rise time profiles with full resolution CIs at target flow rates of 30, 60 and 90 L/min and with orifice plates representative of high-, medium-, and low-resistance DPIs. The study goal was to quantify how such differences may affect characteristic flow rate-rise time profiles.
Presented at Drug Delivery to the Lungs (DDL) 2022, Edinburgh, UK
1 Jolyon Mitchell Inhaler Consulting Services Inc., 1154 St. Anthony Road, London, N6H 2R1, Canada
2 Applied Particle Principles LLC, 17347 Westham Estates Court, Hamilton, VA 20158, USA
3 Mechanical and Manufacturing Engineering, Loughborough University, Loughborough, LE11 3TU, UK.
4 Kindeva Drug Delivery, Derby Road, Loughborough, LE11 5SF, UK
5 Copley Scientific Ltd., ColwickQuays Business Park, Rd. No 2, Colwick, Nottingham NG4 2JY, UK
6 Sofotec GmbH, a member of the AstraZeneca Group, Benzstraße1-3, Bad Homburg, D-61352, Germany