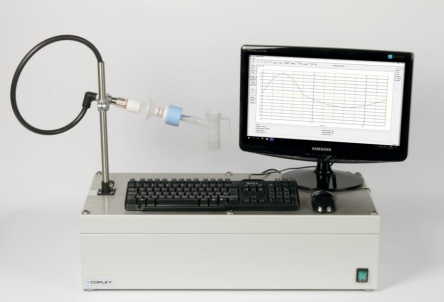
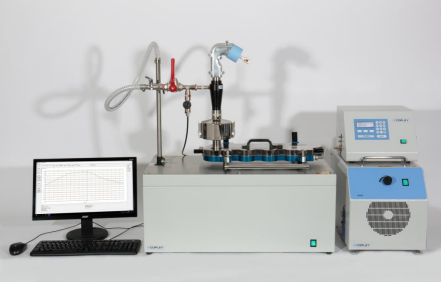
13 November 2017; Nottingham, UK: Copley Scientific, the world’s leading manufacturer of inhaler test equipment, launches new advanced breath simulators, the BRS 2100 and BRS 3100, for orally inhaled product (OIP) development and testing. In recent years, in response to regulatory and pharmacopoeial changes, and the drive to ensure closer correlation between in vivo and in vitro performance, scientists have increasingly incorporated breath simulators into their testing methods. Now, the upgraded BRS simulators from Copley Scientific make it even easier to apply specified inhalation profiles during OIP testing.
The BRS 2100 is ideally configured for testing nebulisers and metered dose inhalers (MDIs) with spacers/valved holding chambers (VHC) in accordance with US Pharmacopeia Chapter <1601> and Chapter <1602> respectively; the latter having only recently been released. The BRS 2100 has a maximum volume of 900 mL making it ideally suited to the low-volume, tidal breathing applications required by these chapters. The BRS 3100 offers greater volumes (500 mL up to 5000 mL) and the increased power (max flow rate of 240 L/min) needed for generating profiles typical of patients using MDIs and dry powder inhalers (DPIs) under forced inhalation conditions, helping to improve in vitro-in vivo correlations (IVIVCs).
As well as generating the ‘standard’ breathing profiles needed to measure delivered dose uniformity (DDU) according to pharmacopoeial requirements, namely: neonate, infant, child, and adult, for nebulisers and MDIs with spacers/VHCs, both the BRS 2100 and 3100 are designed to generate user-defined, patient-derived breathing profiles that more closely mimic real-world clinical situations. Importantly, the standard profiles can be used as a base and edited for development studies, adjusting the selected square, sinusoidal or triangular pattern, the tidal volume and the number, duration and timing of each breathing cycle, for example. For even more control, users can build and then save their own unique breathing profiles to simulate any desired pattern or breathing technique, as may have been recorded in clinic. Both breath simulators can be used for DDU applications or combined with a cascade impactor, using a mixing inlet, for aerodynamic particle size distribution (APSD) studies under more patient realistic conditions.
Compared with prior BRS 2000 and 3000 models, the BRS 2100 and BRS 3100 both feature an upgraded Quad-Core processor, increased RAM, improved Windows 10 operating system, and redesigned software, as part of the embedded, on-board computer. Significant gains in data processing speed and usability are the result. The instruments are more flexible too, for example, flow rates can be displayed in mL/s or L/min, and it is now easier to simulate uncoordinated product use, in the case of VHCs, by synchronising sampling with the exhalation portion of a breathing profile. In addition, the mechanics have been advanced – with improved motor, piston and cylinder performance enabling the more rigorous testing of high-resistance inhalers, and the creation of more powerful inhalation profiles. Additional peripherals and external data transfer are improved by the increased number of USB ports.
Mark Copley, Sales Director at Copley Scientific, comments:
“In recent years, breath simulators have become a core piece of OIP testing equipment. This is partly because of the need to achieve better in vitro-in vivo relationships to enhance product development, and also in response to changing regulatory guidance, which has been updated for greater clinical relevance. Significant progress was made when the previous generation of breath simulators were introduced and now, with our upgraded series of instruments, we are very pleased to be putting state-of-the-art tools once again into the hands of our users.”
To find out more about the BRS range or the company’s complete portfolio of equipment for inhaled drug testing, contact Copley Scientific at sales@copleyscientific.co.uk or download ‘Quality Solutions for Inhaled Testing’ from http://www.copleyscientific.com/downloads/brochures
CAPTION: The new BRS 2100 set up for DDU testing of nebulisers (top) and a test set-up optimised for improved IVIVCs, incorporating the BRS3100 and the adult Alberta Idealised Throat (bottom).
About Copley Scientific
Copley Scientific is recognised as the world’s leading manufacturer and supplier of inhaler test equipment and is a major provider of testing systems for other pharmaceutical dosage forms. The company is also active in detergent testing.
Copley Scientific’s pharmaceutical product range includes test equipment for delivered dose uniformity and aerodynamic particle size measurement of metered-dose inhalers, dry powder inhalers, nebulisers and nasal sprays; as well as tablets (dissolution, disintegration, friability and hardness) capsules, powders, suppositories, semisolids and transdermals.
Copley Scientific has offices in the UK and Switzerland and works in partnership with aerosol particle science experts MSP Corporation in North America.
Serving the pharmaceutical and detergent industries, Copley Scientific offers an extensive range of equipment for research, development and quality control, as well as full validation and aftersales services. This broad range of products is supplied and supported worldwide through a network of specialist distributors.