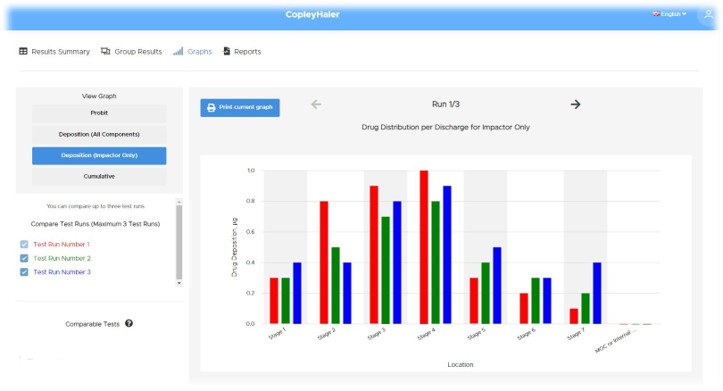
Watch our on demand webinar, where we revealed the latest updates to Inhalytix, the ultimate software solution for Orally Inhaled and Nasal Drug Product (OINDP) data management. Designed by Copley, world leaders in OINDP testing applications, Inhalytix offers a robust, flexible, and fully validated platform tailored to streamline the entry, analysis, and reporting of impactor data and Aerodynamic Particle Size Distribution (APSD) metrics.
Discover the recent software updates that have transformed Inhalytix into an even more powerful tool for inhaler testing analysts worldwide. Explore the new features, including the capability to analyse multiple APIs in a single test to effortlessly handle multi-active products, and input shot weight data directly for greater accuracy and precision.
Whether you’re new to APSD data management or looking to optimise your current strategy, these updates will revolutionise your OINDP testing workflow.
Watch the recording here
About the Presenter
Dr Clair Brooks | Applications Specialist
As an accomplished life sciences professional, Clair has extensive experience supporting the start-up and operation of heavily regulated testing labs across both industry and academia. Offering in-depth application support to those working with orally inhaled and nasal drug products (OINDPs) and other pharmaceutical dosage forms, Clair helps analysts ensure regulatory compliant testing and advises on how best to apply Copley products and services to help maximise return on investment.