Improving IVIVCs
Please select an an item from below for further information about Improving IVIVC’s.
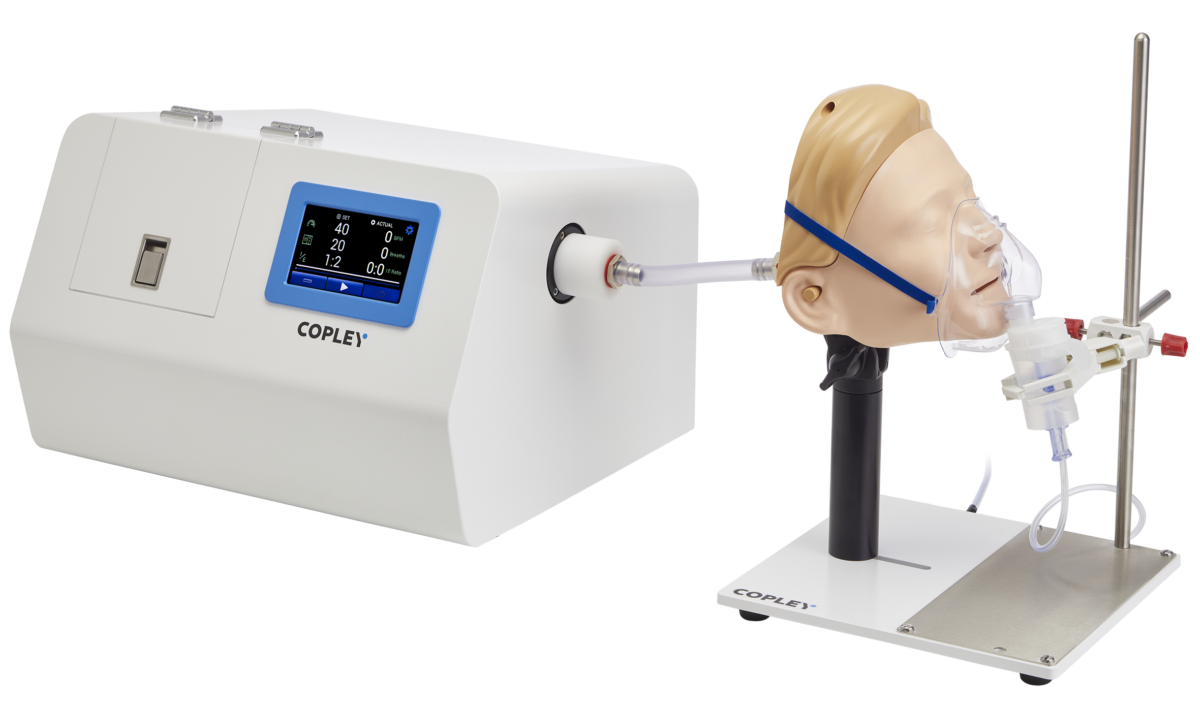
IVIVC Test Systems for DDU and APSD
Two factors have been identified as being critical to improving the clinical relevance of DDU testing and APSD measurement: realistic breathing profiles and realistic throat and nasal models.
View More Details
Inhaled Dissolution
In the case of inhaled and nasal drug delivery products, once the drug is deposited to the target site, the absorption or lung uptake, and hence the therapeutic effectiveness of the drug, depends on the active dissolving in the small amounts of aqueous fluid and lung surfactant available.
View More Details
Facemask Testing
One of the main factors influencing the amount of inhaled drug available to the patient is the interface between the facemask and the patient. Investigating and defining the effect of a facemask to the product’s DDU and APSD is important for the product development.
View More Details
Morphology
Whilst cascade impaction provides a useful indication of where inhaled drug particles are likely to deposit within the respiratory tract, it does not profile the morphological properties of these particles.
View More Details
Cold Freon® Effect
Cold Freon®” effect is the inadvertent reaction to the chilling sensation that hits the back of the throat following actuation of usually MDIs or Nasal devices.
View More Details


Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out More