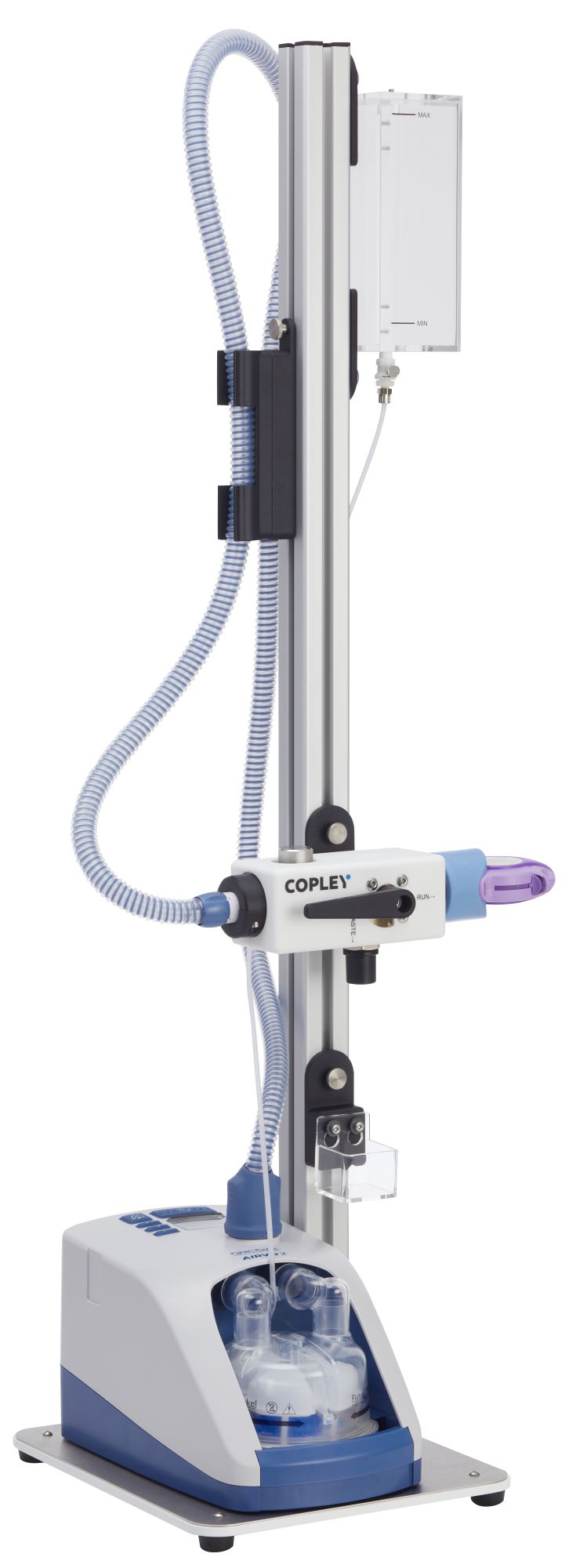
Patient Exhalation Simulator (PES)
Exhalation into the inhaler mouthpiece prior to the inhalation step is a commonly reported error in patient technique associated with inhaler use. For Dry Powder Inhalers (DPIs), exhaling into the mouthpiece may result in the dose clumping and adhering to the inner mouthpiece surfaces, compromising drug delivery to the patient.
Investigating the impact of exhalation through a device complements current EMA/ISO guidance on understanding device robustness and evaluating performance under conditions simulating patient use.
Replicating the effects of a patient exhaling into a device mouthpiece prior to the inhalation step, the Patient Exhalation Simulator (PES) enables developers to assess how device misuse impacts the CQAs of the inhaler, to empower device design optimisation and ensure robust drug delivery.
Simple to use
Easy to set-up, with minimal maintenance required
Ideal for assessing a wide range of patient profiles


Air flow to inhaler: Exhalation simulations are performed by directing warm, humid air at the inhaler mouthpiece for a set period of time.
Air flow to waste: At the end of the timed exhalation, the air flow is directed to ‘Waste‘ via a manually controlled valve.
Patient Exhalation Simulator PES: Technical Specifications | |
Temperature set-points: | 31°C, 34°C, 37°C |
Humidity set-points: | Always saturated |
Flow rate set-points: | 10 – 60 L/min |
Dimensions (w x d x h): | 225 x 300 x 1030 mm |
Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out More

