Contact Us
Contact Us
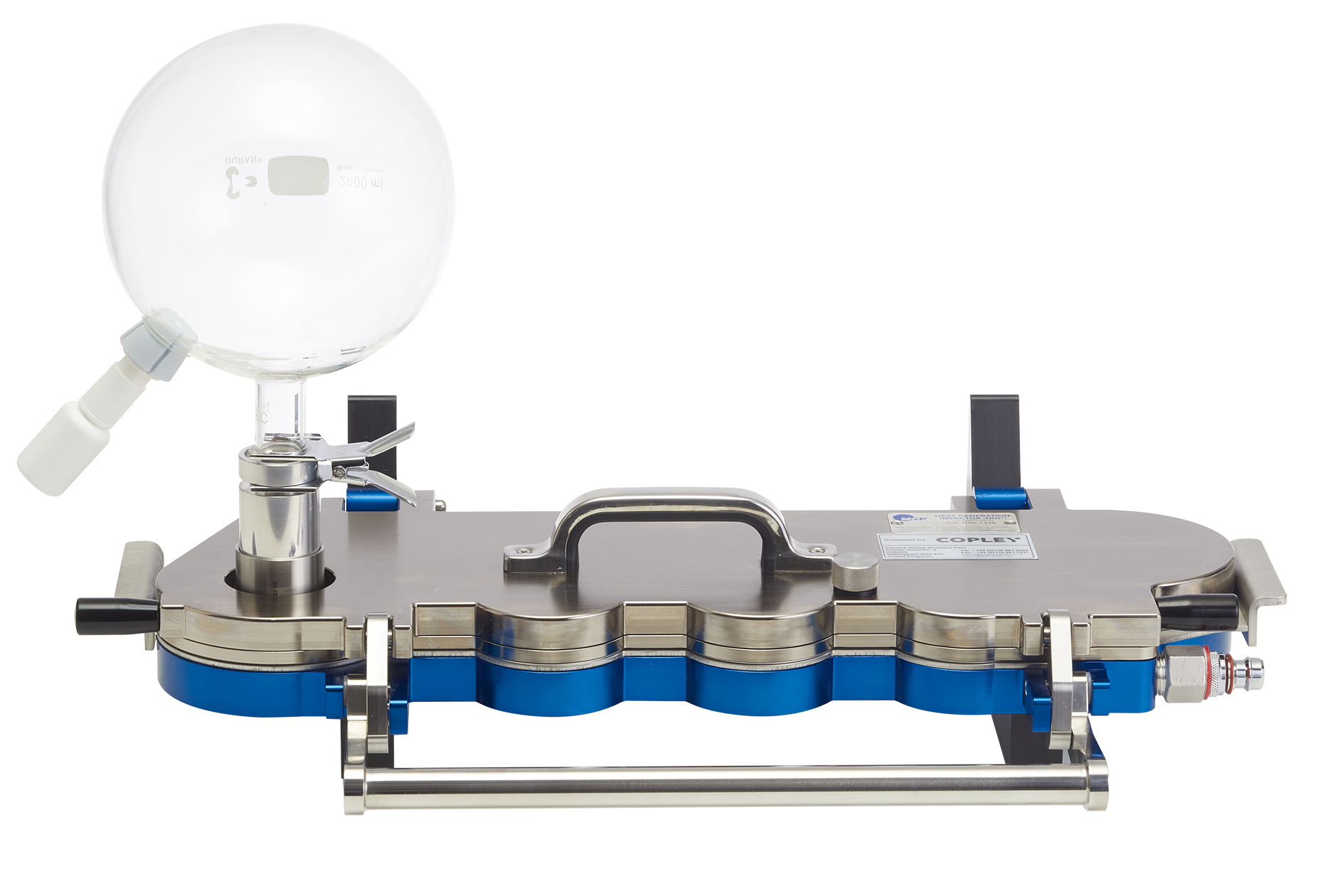
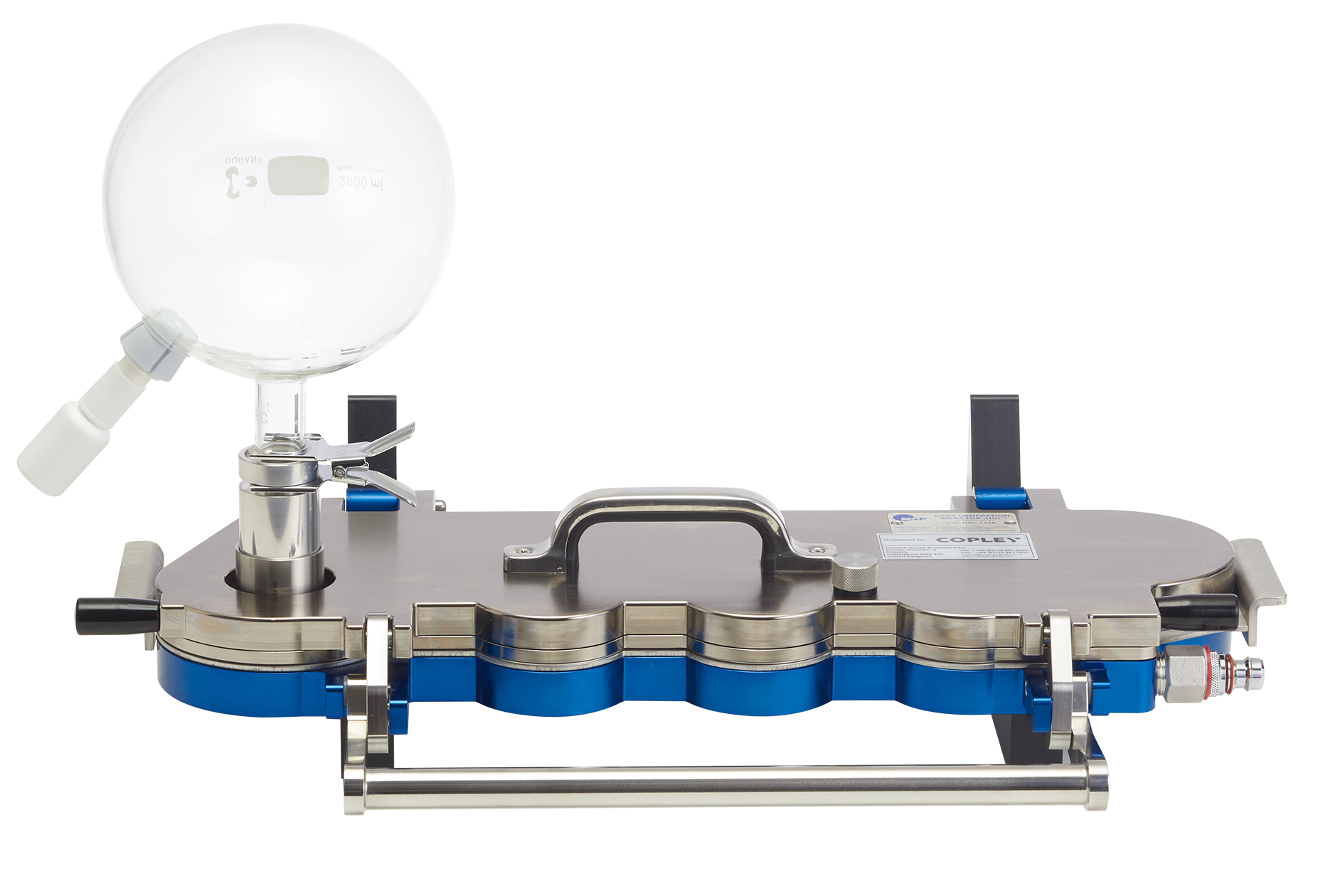
Glass Expansion Chambers
The use of a cascade impactor together with a high volume expansion chamber is used to measure the amount of drug in small particles or droplets in respect of nasal sprays and aerosols. In accordance with the draft guidance, we offer a range of glass expansion chambers to meet these requirements. We offer 3 sizes:
1 L chamber: to maximise drug deposition below the top stage of the impactor (i.e. for nasal aerosols)
2 L chamber: to maximise aerosolisation and impactor deposition (i.e. for nasal sprays)
5 L chamber: for powerful nasal sprays where increased space is required to generate full plume
Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out More