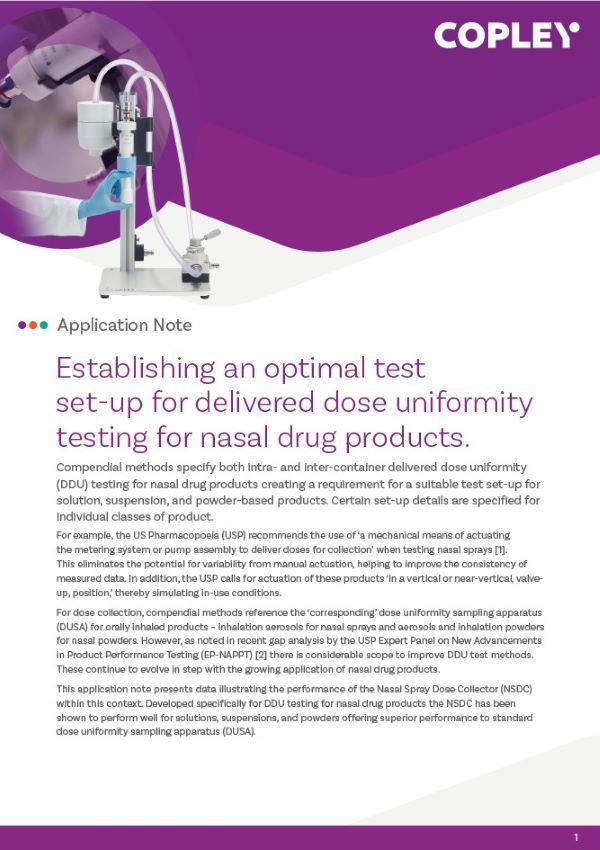
Home > Inhaler Testing > Delivered Dose Uniformity (DDU) > DDU of Soft Mist Inhalers (SMIs)
DDU of Soft Mist Inhalers (SMIs)
Both MDIs and DPIs suffer from the same two inherent problems: low lung deposits (typically 5-20%) and dose variability (often due to patient difficulties in coordination or inspiration).SMIs (often known as “Inhalation Metered Sprays’’ or “Aqueous Droplet Inhalers”) actively aerosolise the liquid, forming a ‘soft mist’ to overcome these problems. These inhalers generally deliver a higher fine particle fraction than MDIs or DPIs. However, as with any multi-dose liquid system, microbial contamination can be a problem. Since they are active, aqueous-based devices, the DDU testing of ADIs is similar to that of MDIs, with testing carried out at a constant flow rate of 28.3 L/min.



Contact Us
Contact Us
Enquire about DDU of Soft Mist Inhalers (SMIs)
Low Capacity Pump LCP6
View BrochureBreath Actuation Controller BAC 100i
View BrochureInhaler Testing Workstation ITW
View ProductDose Uniformity Sample Apparatus (DUSA) for MDIs
View BrochureFlow Rate Sensor FRS
View BrochureWaste Shot Collector WSC2
View BrochureMouthpiece Adapter
View ProductEnviroMate™ – Benchtop Environmental Chamber
View BrochureFlow Meter DFM 2000
View BrochureDUSA Stand for DPIs
View BrochureTemperature/Humidity Sensor
View BrochureLabel Printer
View BrochureAPSD of Aqueous Droplet Inhalers (ADIs)
View SystemLow Capacity Pump LCP6
View BrochureBreath Actuation Controller BAC 100i
View BrochureInhaler Testing Workstation ITW
View ProductDose Uniformity Sample Apparatus (DUSA) for MDIs
View BrochureFlow Rate Sensor FRS
View BrochureWaste Shot Collector WSC2
View BrochureMouthpiece Adapter
View ProductEnviroMate™ – Benchtop Environmental Chamber
View BrochureFlow Meter DFM 2000
View BrochureDUSA Stand for DPIs
View BrochureTemperature/Humidity Sensor
View BrochureLabel Printer
View BrochureAPSD of Aqueous Droplet Inhalers (ADIs)
View SystemDDU Over the Entire Contents
In the case of multiple dose devices, tests might need to be carried out throughout the life of the inhaler i.e. dose uniformity over the entire contents.
Learn MoreRelated Applications
We also offer a range of equipment for additional MDI testing application support:
Related Applications
Two factors have been identified as being critical to improving the clinical relevance of DDU testing and APSD measurement: realistic breathing profiles and realistic throat and nasal models.
View More
Semi-Automation Tools
Semi-Automation Tools
All of the tests involved in Delivered Dose Uniformity require the collection and drug recovery of individual doses into the collection tube of a Dosage Unit Sampling Apparatus (DUSA) appropriate to its type (MDI or DPI) prior to assay.
View Product
Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out MoreRelated News & Resources

Have a question? Our friendly and experienced team are here to help.