
Centralised Control for APSD Data Analysis
One Platform, Complete Oversight
Inhalytix®+ brings together all critical elements of APSD data management into one connected system. Test methods, user activity, equipment details, operational data and analytical results are captured and stored in a single, structured environment.
This centralised approach ensures greater visibility, consistent data handling and easier access to the information that matters. Whether managing workflows, ensuring traceability or supporting compliance, Inhalytix+ helps streamline operations across the entire testing process.
Designed to Simplify Complex Testing Workflows

Unified APSD Workflow
Bring method setup, equipment connectivity, test execution, and results reporting into one connected platform. Inhalytix+ simplifies the entire APSD process by reducing manual input and enabling a consistent, structured approach.

Instant, Reliable Results
Automated data transfer from connected equipment eliminates the need for manual entry and reduces the risk of error – allowing faster, more accurate metric calculations.

Built-In Data Integrity Controls
Every result is verified through integrated validation and structured compliance features, helping ensure accuracy and meet regulatory standards.

Full Traceability
All test results are automatically linked to the method, equipment configuration, and operator responsible. This creates a clear, audit-ready record for every test.


Aligned with Global Pharmaceutical Regulatory Standards
Inhalytix®+ is designed to meet the requirements of major international pharmacopoeias and regulatory authorities, including Ph. Eur., EMA, USP, FDA, JP, CFDA and ChP. This alignment helps laboratories ensure that APSD data meets global regulatory standards from the very start.
By combining compliance-focused features with a streamlined digital workflow, Inhalytix+ supports efficient, accurate testing while maintaining full adherence to current regulatory expectations. Built-in controls, audit trails, and structured approvals make it easier for teams to work confidently in regulated environments.


Dashboard
Instant Insight into Your Operation
Start every session with a clear picture of your lab’s activity. The Inhalytix®+ dashboard displays real-time data on active analysts and supervisors, the number of tests prepared, executed, and completed, and an overview of your equipment and report configurations. An integrated, type-organised equipment inventory puts asset details front and centre, helping you stay on top of usage, performance, and availability.
Equipment Inventory
Centralised Traceability and Control
Managing inhaler testing assets has never been easier. The Inhalytix®+ equipment library centralises your inventory and links each asset directly to the relevant test data and reports. Maintain complete traceability of which instruments were used for each test, and enhance precision with impactor-specific mensuration data for accurate stage cut-off calculations.
Equipment Bench
Total Traceability at Your Fingertips
Managing inhaler testing assets has never been easier. The Inhalytix®+ equipment library centralises your inventory and links each asset directly to the relevant test data and reports. Maintain complete traceability of which instruments were used for each test, and enhance precision with impactor-specific mensuration data for accurate stage cut-off calculations.
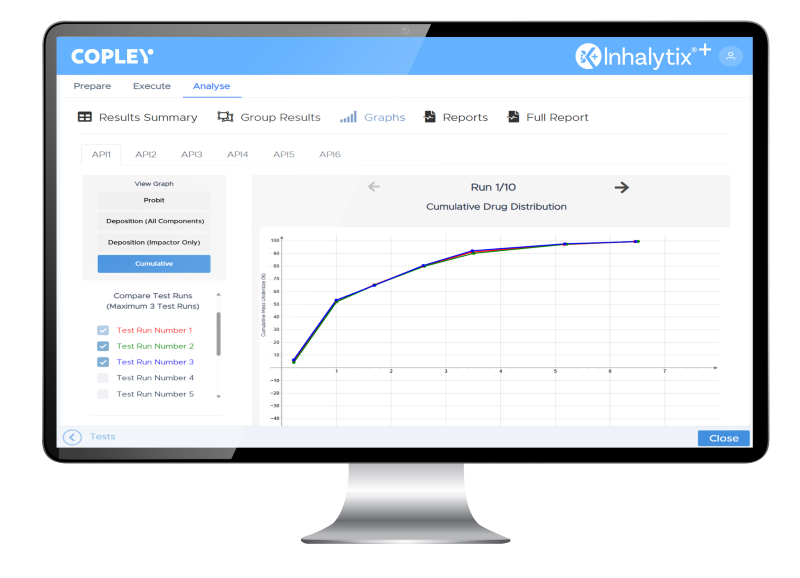
Data Analysis
Fast, Reliable Data Interpretation
After data entry or import, Inhalytix+ processes information with advanced algorithms to present results in multiple formats. Key metrics are displayed in a scrollable Results Summary, drug fractions appear in Groups Results, and graphical views include log-probit plots, drug deposition, and cumulative distribution charts. Comparison of up to three test runs is supported, along with generation of both standard and custom reports.

Simple, All-Inclusive Subscription
Inhalytix®+ is offered as an all-inclusive subscription that provides everything your lab needs for efficient and compliant APSD testing.
Your subscription includes:
✔ Unlimited test run analyses
✔ Continuous updates to maintain compliance and add new features
✔ Built-in audit trails for complete traceability
✔ Customisable methods and reporting capabilities
✔ Comprehensive technical support
✔ Optional user training packages to help your team get up to speed
Software Updates
We have recently released a new version (2.4.0) of our legacy Inhalytix software product. To check if your software version is out of date and may be missing critical updates and bug fixes, please contact support@copleyscientific.co.uk to ensure your data integrity and discuss updating to the latest version as soon as possible.
Notification of Withdrawal of Support for CITDAS
Formal notification of the withdrawal of technical support for all versions and releases of CITDAS was effective 30th November 2023. This means, Copley will no longer provide help for bug fixes, error messages, custom work or any other issues. Copley launched the Inhalytix software application in 2021 which effectively superseded CITDAS. Inhalytix provides the capabilities of CITDAS alongside other advanced features, and has been verified to provide the same APSD metrics when the same raw data is input into both applications.





