31 May 2018; Nottingham, UK: New accessories from Copley Scientific simplify the rigorous investigation of how a facemask impacts potential critical quality attributes of the delivered dose from a nebuliser, enhancing the clinical realism of Quality by Design and bioequivalence studies. Prescribing clinicians routinely use facemasks in place of a mouthpiece to ease nebuliser use, especially for children or the infirm, but this can significantly impact drug delivery. The new Facemask Test Stand (FMS) and Next Generation Impactor (NGI) to FMS Interface Accessory make it straightforward to extend existing pharmacopoeia methods to investigate this effect.
Current pharmacopoeia methods for delivered dose uniformity (DDU) and aerodynamic particle size distribution (APSD) measurement for nebulisers, as specified in USP Chapter <1601> and Ph. Eur. 2.9.44, are based on the use of mouthpieces. However, the use of facemasks has the potential to alter both DDU and APSD. Testing with a facemask in situ is therefore advantageous both for rigorous, clinically realistic performance assessment and for product comparison (test to reference), and mirrors the approach already specified for spacer/valved holding chamber (VHC) equipped metered-dose inhalers (MDIs) in USP Chapter <1602>.
The FMS can be used with the full range of Copley face models – infant, child or adult – or with a user-validated alternative, as required. It enables secure interfacing of the nebuliser, with facemask in place, with the standard DDU filter holder apparatus specified for nebulisers. The corresponding test set-up for APSD measurement additionally uses the NGI to FMS Interface Accessory to ease connection of the nebuliser, via the facemask (except infant), to the NGI, which is typically the impactor of choice for nebuliser testing. In combination the two accessories support the efficient generation of DDU and APSD data for nebulisers, with a facemask in place.
To find out more about the FMS, FMS to NGI accessory or any other aspect of nebuliser testing contact: sales@copleyscientific.co.uk
To see Copley’s full range of equipment for inhaled drug testing download “Quality Solutions for Inhaled Testing” from: http://www.copleyscientific.com/downloads/brochures
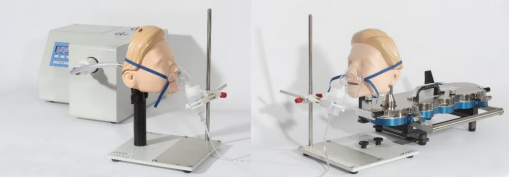
CAPTION: Measuring the critical quality attributes of a nebuliser dose with a facemask in place – delivered dose uniformity (left) and aerodynamic particle size distribution (right) – improves the clinical realism of test data.
About Copley Scientific
Copley Scientific is widely recognised as the world’s leading manufacturer and supplier of inhaler test equipment and is a major provider of testing systems for other pharmaceutical dosage forms. The company also supplies equipment for detergent testing.
Copley Scientific’s pharmaceutical product range includes test equipment for all types of orally inhaled and nasal drug products – metered-dose inhalers, dry powder inhalers, nebulizers and nasal sprays – with a particular focus on solutions for delivered dose uniformity and aerodynamic particle size distribution measurement. It also includes testers for tablets (dissolution, disintegration, friability and hardness) capsules, powders, suppositories, semisolids and transdermals.
Used from R&D through to QC, this extensive range of equipment is supported by a full validation and aftersales service. Copley Scientific has offices in the UK and Switzerland and works in partnership with aerosol particle science experts MSP Corporation in North America; specialist distributors extend localised support across the world. This network provides expert help and training to every customer, directly enhancing the application of all Copley Scientific products.







