What are semisolids, how do they work and how are they formulated? Establishing the foundations for a better understanding of testing requirements.
For the average adult, the surface area of the skin is 1.5 – 2.0 m². That’s a large area for drug delivery but one with impressive defences against the ingress of unwanted substances.
In a previous blog, we explored transdermal patches. Today we’re turning our attention to semisolids, a versatile alternative vehicle for dermal drug delivery. This introductory Q&A outlines what semisolids are, how they work, how they’re formulated, and what you need to know about their testing requirements.
What are semisolids?
Semisolids is an umbrella classification that covers therapeutic creams, lotions, ointments, gels and foams. Often referred to as topicals, these are typically oil-in-water or water-in-oil emulsions with the active pharmaceutical ingredient (API) dissolved in one of the phases. Most offer localised action, treating disorders of the upper layers of the skin but not all. There are semisolids for subcutaneous and systemic action as we’ll discuss later.

Semisolids are especially valued for topical drug delivery to the skin and enjoy high patient acceptability.
The use of semisolids dates back centuries. Galen’s cold cream, a beeswax, olive oil and (rose) water emulsion, developed in 150 AD is one of the earliest examples. Today’s formulators have a wide range of synthetic hydrocarbon ingredients at their disposal, but natural products such as lanolin remain commonplace. Functional excipients that, for example, enhance stability, rheological properties and/or penetration are also now routinely part of the mix.
Why are semisolids so widely used?
Primarily because they have attractions for both drug developers and for patients:
For drug developers:
- Localised delivery: semisolids are especially suitable for the treatment of skin conditions, allowing drug delivery directly to the site of action. This is efficient with respect to dosing and enables high localised drug concentrations, where necessary, with few side effects; undesirable systemic uptake is typically minimal.
- Formulation flexibility: since dryness is a feature of many skin conditions the opportunity to simultaneously formulate for rehydration, for symptomatic relief and to restore skin functionality, is also valuable.
- Bypassing first-pass metabolism: dermal delivery shares with other parenteral routes the advantage of avoiding first pass metabolism and the variabilities of gastro-intestinal absorption.
For patients:
- Ease of application: semisolids are convenient and easy-to-use
- Rapid symptom relief: in many instances, products offer immediate as well as long-term relief from troublesome symptoms.
- Minimal side effects: since systemic exposure tend to be low.
Correspondingly high patient compliance brings benefits for all stakeholders – drug developers, healthcare practitioners and patients alike.
What drugs are they used for?
Anti-infectives and topical corticosteroids are two of the most common classes of drug formulated as semisolids , but antibiotics, antivirals and retinoids (vitamin A-related products that boost skin renewal and collagen) are all widely used. Acne, tinea infections, psoriasis, and atopic dermatitis top the list of applications i. Other conditions routinely treated with locally acting gels and creams range from conjunctivitis (a disorder of the eye) to oral ulcers and gingivitis (inflammation of the gums).
Beyond products for localised action, analgesic gels are an important focus, as exemplified by formulations incorporating non-steroidal anti-inflammatory drugs (NSAIDs) for the treatment of joint pain. Here, dermal delivery reduces the risk of negative impacts on the gastro-intestinal tract, providing a good, long-term solution for pain relief for those suffering from diseases such as arthritis. Systemic delivery of hormones is also a key application with dermal delivery again offering lower risk relative to oral delivery for certain patients.
What are the key formulation challenges?
- Controlling the release profile: achieving a predictable and therapeutically effective drug release rate is a key task for semisolids formulators. This determines both the onset and duration of action. Both are critical quality attributes for semisolids.
- Product stability: the emulsified nature of semisolids makes them prone to separation so functional excipients may be necessary to achieve an acceptable shelf life and safeguard dose uniformity.
- Optimising rheology: Rheological properties (flow behaviour) influence application characteristics and patient experience such as how easily the product can be smoothed across the skin. Formulators optimise rheology to balance ease-of-use with stability.
- Overcoming the skin barrier: For subcutaneous or systemic effects, drug molecules must pass through several protective layers. The skin presents substantive physical, chemical, microbial and immunological barriers to the ingress of potentially damaging agents. These limit systemic exposure and associated side effects for dermatological products but are a major hurdle when targeting joints or muscles, or systemic action.
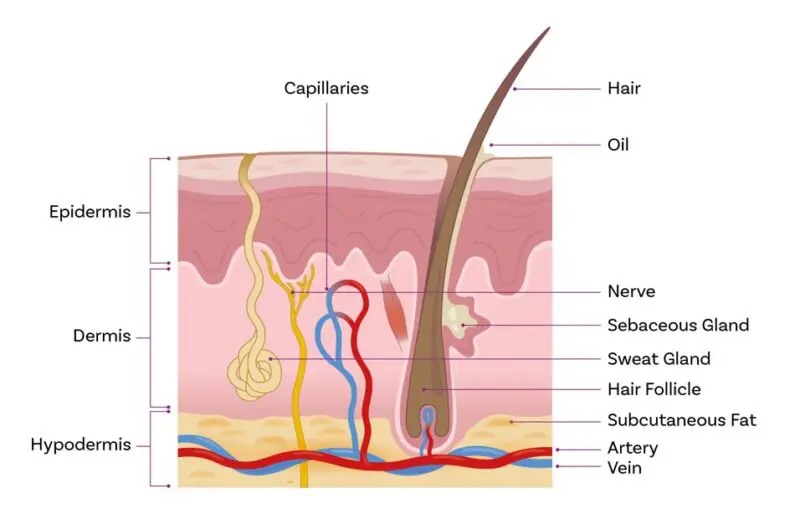
Drug formulators must navigate the complex multi-layered structure of the skin when delivering drugs for subcutaneous and systemic action
The physicochemical properties of the drug determine its ability to penetrate the skin, the easiest candidates being small/low molecular weight, lipophilic molecules that are effective at low concentration. Oestrogens are a prime example. However, formulators have a range of strategies at their disposal for more challenging drugs. Topical NSAIDs, for example, are typically formulated as high-water content gels with chemical penetration enhancers such as isopropyl alcohol, propylene glycol and dimethyl sulfoxide to ensure efficacy ii.
What compendial chapters/regulatory guidance are most relevant?
- USP Chapter <3> Topical and Transdermal Drug Products covers all dermal drug delivery vehicles. It specifies the measurement of physicochemical properties such as pH and particle size, and specifically for semisolids, apparent viscosity and dose uniformity.
- USP Chapter <1724> Semisolid Drug Products – Performance Tests, updated in December 2023, focuses on In Vitro Release Test (IVRT) and In Vitro Permeation Test (IVPT) studies. For many years there were no compendial methods specifically for semisolids, but this chapter now includes substantial detail on diffusion cell test methods for performance assessment.

Modern diffusion cells are an easy-to-use solution for compendial semisolid testing
The FDA offers over 80 Product-Specific Guidances (PSGs) for semisolids, to support ANDA submissions iii. And this number continues to grow. Recent updates include a PSG for adapalene, benzoyl peroxide and clindamycin phosphate gels, along with a revised version of the PSG for azelaic acid (both November 2024) iv .
The EMA provides generalised guidance for semisolid submissions v.
How does testing support formulation and approval?
In vitro compendial methods for semisolids help formulators to manipulate composition and processing steps towards desirable product quality and performance or, in the case of generics, towards sameness. Focusing on performance testing, IVRT characterises steady-state drug release rate from the product matrix while IVPT elucidates drug permeation through the skin under biorelevant conditions. Both are highly relevant when it comes to controlling clinical efficacy.
Of course, these tests not only support formulation but also enable effective product QC. Furthermore, for generics they enable a route to regulatory approval with no requirement for in vivo studies. The importance and value of establishing optimal in vitro test set-ups should therefore not be underestimated. These methods are critical and valuable across the whole semisolids lifecycle.
Looking Ahead
This blog lays the groundwork for understanding semisolid formulations and their testing landscape. In a future post we’ll delve deeper into IVRT and IVPT methodologies, with practical insights into experimental setup, regulatory expectations, and data interpretation.
If you found this overview helpful, make sure to subscribe to our blog to be notified when the next post in this series is published.
In the meantime if you are interested in learning more about the tools and technologies used in semisolid testing, then learn more about the diffusion cells we offer for this important application.
i J.R. Walter and S. Xu ‘Topical Drug Innovation from 2000 Through 2014’ Research letter. JAMA Dermatol. 2015;151(7):792-794.
ii ML. McPherson and N.M. Cimino ‘Topical NSAID Formulations ‘Pain Medicine’ Vol 14 Issue suppl_1. Dec 2013 S35-S39
iii FDA ‘An Overview of the Current Product-Specific Guidances for Topical Products’ Presentation delivered at SBIA 2023—Advancing Generic Drug Development: Translating Science to Approval. 13th Sep 2023
iv FDA Product-Specific Guidances for Generic Drug Development. Available to view at: www.accessdata.fda.gov/scripts/cder/psg/index.cfm
v EMA Committee for Medicinal Products for Human Use ‘Guideline on quality and equivalence of locally applied, locally acting cutaneous products’ 9th Sep 2024.