A guide to options for measuring the extent and rate of drug dissolution, Critical Quality Attributes (CQAs) for oral solid dosage (OSD) forms.
In a previous blog we discussed the centrality of dissolution testing for OSD forms and the basic principles of how it’s done. Today, we’re going to dig deeper into the different types of apparatus used, as detailed in the pharmacopoeias (specifically, Ph. Eur. 2.9.3, USP <711>, and JP 6.01).
Why are there are so many different designs of dissolution apparatus – five for OSDs, seven if you include transdermals?
And which should be used for which type of OSD?
Read on to find out…
To Begin: Apparatus 1 – Basket
Apparatus 1 was first adopted for use in compendial dissolution testing in 1970i. Today, it retains its foremost position, accounting for the majority of dissolution testing along with Apparatus 2.
The illustration below shows Apparatus 1. The sample is held in a basket that rotates within a vessel containing a significant volume of dissolution media (standard vessel volume is 1000 mL). Basket mesh size can be varied to meet individual product requirements with multiple options available alongside the standard (40 mesh) design.
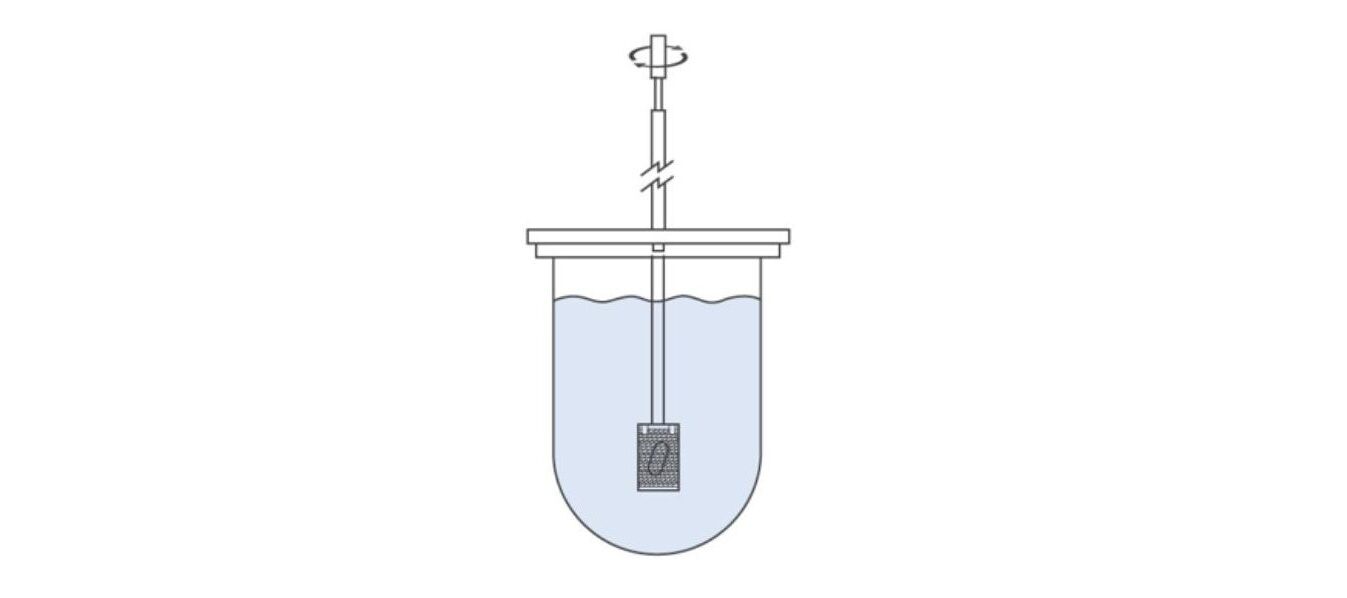
Apparatus 1 is well-suited to capsules, tablets, chewables, and extended-release formulations, and to encapsulated beads. However, it is less than optimal for tablets that release fine powders during disintegration since by escaping from the basket these becomes subject to a shift in hydrodynamic regime.
Addressing the Issue of Fine Particles: Apparatus 2 – Paddle
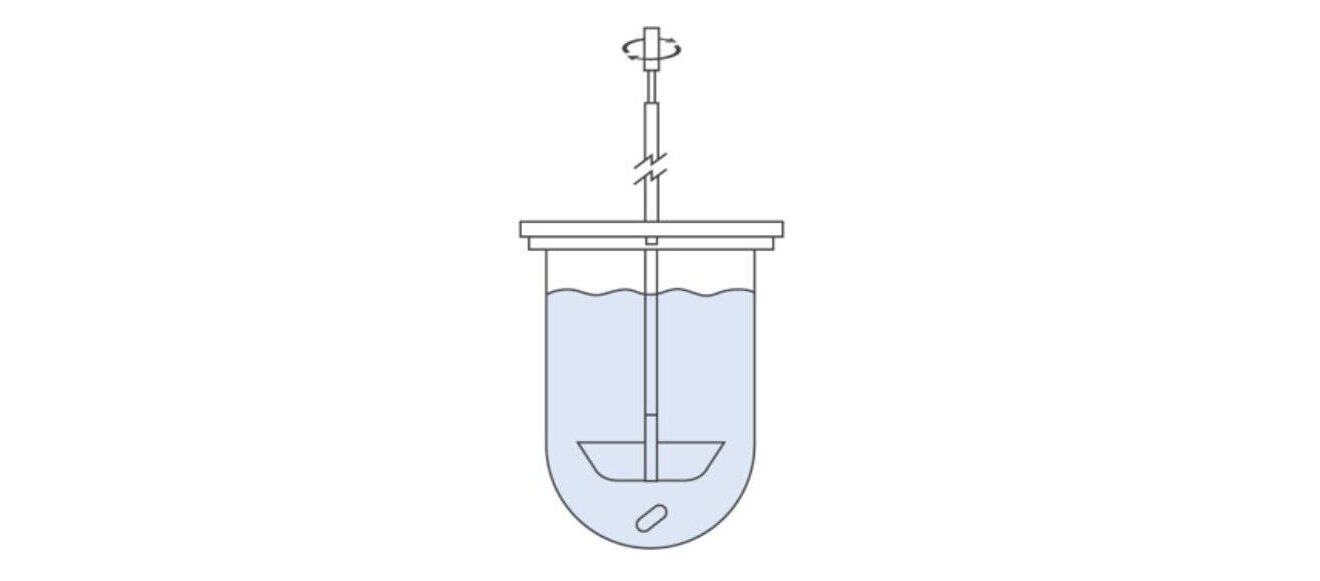
The illustration above shows Apparatus 2. The use of a paddle in place of a basket avoids the issue of fine particles transitioning into a different dissolution regime but Apparatus 2 is otherwise identical to Apparatus 1 with many commercial units enabling direct interchangeability between the two. As well as being suitable for the same OSD forms, it is specifically useful for suspensions, tablets made by the direct compression of fine blends, and powder-filled capsules.
The testing procedure with both Apparatus 1 and 2 is straightforward, as outlined below:
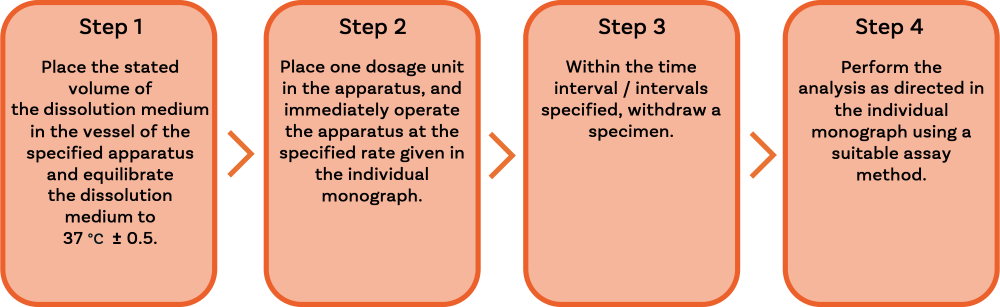
And they share the following key features, all of which directly influence precision and accuracy:
- Precisely dimensioned test vessel
- Metallic drive shaft to carry the basket/paddle
- Motor for rotation of the basket/paddle at the required speed
- Water bath, heater, and associated control loop to maintain a consistent temperature
- Fitted cover to reduce evaporation of the dissolution media.
Speed of rotation of the basket/paddle, the dimensions of the test vessel, and of the basket (where used, including mesh size) influence the hydrodynamic regime to which the dose is subjected, defining the flow pattern at the liquid/solid interface between solvent and drug. Precision on all fronts is therefore vital for repeatability. The use of covers to reduce evaporation is also about precision but with respect to media volume. Drug assays determine drug concentrations which are converted into meaningful dissolution data via test media volume. Changes or inaccuracies in test media volume are therefore a source of error, notably for poorly soluble drugs. Finally, temperature control is essential for both repeatability and clinical relevance since drug solubility is directly dependent on temperature. The pharmacopoeias indicate a test temperature of 37oC +/- 0.5oC for testing OSD products, reflecting in vivo conditions.
These two apparatuses are prized for their simplicity, ease-of-operation, and robustness but they also share some limitations. For example, it’s not easy to realistically simulate transit through the gastrointestinal tract and the hydrodynamic regime is not strictly controlled. Issues such as shaft location, centring and wobble, and coning, whereby insoluble elements of the tablet collect beneath the basket or cone, can all disrupt hydrodynamics and by extension, impact test results.
Alternative Dissolution Apparatuses for OSD Forms
The schematic below shows the other three test apparatuses specified for OSD forms. Though less routinely deployed than Apparatus 1 and 2, these all have specific benefits and associated applications.
Exploring the Impact of Dissolution Conditions: Apparatus 3 – Reciprocating Cylinder
Apparatus 3 was adopted in 1991 for testing extended release productsi. It was modelled on pre-existing apparatus for disintegration testing to subject the OSD sample to a ‘dipping’ action, the sample floats freely as the inner tube moves downwards and is held against the mesh base on the upward stroke. Reciprocating cylinders are held in water baths in the same way as Apparatuses 1 and 2 to maintain a constant test temperature.
With Apparatus 3 it is easier to simulate the mechanical and physicochemical conditions that influence release in the gastrointestinal tract (GIT) by changing media composition, agitation rate and residence time; the inner sample-containing tube is easily switched between different outer cylinders to facilitate such studies. This makes Apparatus 3 highly suitable for profiling controlled-release products though the small volume of dissolution media it contains can be problematic with respect to maintaining sink dissolution conditions.
Flexible and Versatile: Apparatus 4 – Flow Through Cell
Apparatus 4 enables the consistent maintenance of sink conditions, where required, by subjecting the sample to continuously flowing dissolution media, using a pump to drive the fluid upwards through the cell. The sample is held on a support in the centre of the cell, which in turn is immersed in a water bath or jacket, for precise temperature control.
Apparatus 4 can be operated at different temperatures and flow rates, thereby offering flexibility with respect to the amount of dissolution media that the sample is exposed to. It applies gentle, well controlled hydrodynamic conditions and enables switching of one dissolution media to another, to simulate transit through the GIT.
These characteristics make this method suitable for studying the drug release profile of poorly soluble, modified- and extended-release products, both solid and liquid, oral and non-oral, including medical devices such as implants. That said, there is substantially less experience of this more complex apparatus relative to others and pump precision must be carefully controlled to ensure repeatable data.
Last and Least Volume: Apparatus 7 – Reciprocating holder
Last of the five dissolution testing apparatuses used for OSD products, Apparatus 7 is relatively infrequently used but is well-suited to small volume testing applications including assessment of the impact of a change of media; it is highly amenable to automation. These characteristics make Apparatus 7 useful for osmotic dosage products, and low dose delivery systems (including transdermal patches) which can be robustly assessed using minimal dissolution media volumes.
In Summary
USP Monographs | Principle | Dosage Forms | Medium Volume (mL) | Rotation Speed (RPM) | Temperature | Dip Rate (DPM) |
1 | Basket | Capsules, tablets | 500-4000 | 25-50 | 37°C +/- 0.5°C | – |
2 | Paddle | Capsules, tablets and suspensions | 500-4000 | 25-150 | 37°C +/- 0.5°C | – |
3 | Recoprocating Cylinder | Capsules, tablets, suspensions and granulates | Typically 250 | – | 37°C +/- 0.5°C | 5-60 |
4 | Flow-through Cell | All | Up to 3 L/h | N/A | 37°C +/- 0.5°C | – |
7 | Reciprocating Holder | Transdermal patches, non-disintergrating tablets | Variable | – | 37°C +/- 0.5°C | 5-60 |
A table highlighting the different USP apparatuses and process parameters for tablets, capsules, suspensions and granulates
The range of dissolution testing apparatuses available for OSD products has evolved progressively to address test equipment limitations and in line with formulation requirements. Choosing the best dissolution testing option calls for careful consideration of the characteristics of the dosage form and how it will be used. This is especially true for extended-release products that, in vivo, may dissolve under a range of conditions as they transit through the GIT. The table above summarises the options but whatever the apparatus chosen, the close control of dimensions, hydrodynamics and temperature is crucial to precise, accurate and relevant testing.
In future blogs, we’re going to broaden our discussion of dissolution to other dosage forms. Make sure you sign up today to stay informed and be part of the conversation.
i Uddin, R et al ‘Dissolution and Dissolution Apparatus: A Review.’ Int J Cur Biomed Phar Res 2011; 1(4):201-207








