The first in a three-part series on the importance of improving in vitro in vivo correlation (IVIVC) in the testing of orally inhaled and nasal drug products (OINDPs).
To date we’ve focused on compendial test set-ups for orally inhaled products (OIPs) and nasal drug delivery; now we’re going to look at ways we might choose to modify them.
The development of OINDPs relies on in vitro, in vivo and increasingly, in silico data. As in vitro and in silico tools improve, reliance on more expensive and time-consuming in vivo methods diminishes, a major gain. This has long been the motivation for adopting test set-ups that enhance clinical realism relative to those in the pharmacopoeias.
However, recently the FDA has begun to release Product-Specific Guidances (PSGs) referencing the use of ‘realistic aerodynamic particle size distribution’ (rAPSD) data to streamline product approval. Greater clinical realism in OINDP testing has therefore become mainstream.
In this series of blogs, we’re going to discuss established solutions for greater clinical relevance in OINDP testing. One focus will be breathing profiles, another will be methods for simulating patient behaviour, but to begin, it’s throat and nose models.
Why target greater clinical relevance?
‘A pharmacopoeia is a legally binding collection of standards and quality specifications for medicines used in a country or region.’
This definition is an important reminder of the primary purpose of compendial methods, which is to confirm quality. Measuring performance-defining characteristics such as delivered dose and APSD safeguards adherence to a standard. The associated test methods are ideally simple, repeatable, easy-to-use and validate, and highly differentiating.
Such methods are the cornerstone of product QC but are less optimal for R&D given the difficulty in achieving robust in vitro in vivo correlation (IVIVC). In vivo studies (PK/PD – pharmacokinetic and pharmacodynamic) are part of the answer but these are costly, time-consuming and complex in comparison. And there are other limitations too. PK studies focus on drug concentration in the blood plasma and may therefore be minimally informative regarding bioavailability for a locally acting OINDP. PD studies are complicated by the need to work with a diseased, and by extension, variable, patient population, and can suffer from poor sensitivity.
In silico models are also part of the solution and can help to bridge poor IVIVCs. For example, an effective physiologically-based pharmacokinetic (PBPK) model can predict drug transport around the body from point of entry to excretion, from in vitro measurements.
Improving the clinical relevance of in vitro methods has an important role to play in ensuring the efficient application of each of these tools by:
- Improving in silico results.
- Enabling the refinement of in vivo test programmes to maximise utility.
- Reducing reliance on in vivo data.
And for generic developers, there is added motivation from the test specifications defined in a growing number of FDA PSGs. These support the demonstration of bioequivalence without a clinical endpoint trial via the extension and refinement of in vitro and PK studies. The measurement of realistic APSD with mouth-throat models of different sizes (small and large) is now routinely referenced.
What’s wrong with the USP/Ph. Eur. induction port?
Viewed through the lens of standardised compendial testing, the USP/Ph. Eur. induction port used to interface an OINDP with an impactor has many benefits. It is a simple, easy-to-use inlet with a well-defined geometry that lends itself to precision manufacture and consistent performance.
However, it lacks clinical realism. More specifically, it is common knowledge that the USP/Ph. Eur. induction underpredicts the amount of material that deposits in the upper respiratory tract.
So, what is a suitable replacement to improve clinical relevance?
One option is to use an anatomically correct throat cast. But in truth, these are anatomically correct for just a single subject. The considerable scope for inter-subject variability gives rise to marked variability in deposition behaviour. Furthermore, from a practical perspective throat casts lie at the opposite end of the spectrum to the USP/Ph. Eur. induction port. They are difficult to manufacture, hard to handle and clean, and tend to offer low repeatability.
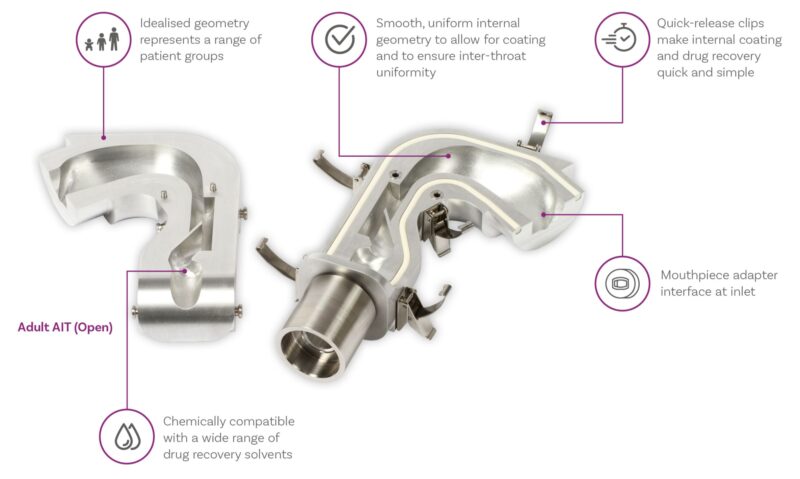
Here’s an alternative – the Alberta Idealised Throat (Adult and Child)
The AIT is the product of extensive research at the University of Alberta. It’s an inlet with a standardised, highly reproducible, human-like geometry, that is easy-to-use and clean, and that delivers greater clinical relevance than the USP/Ph. Eur. induction port.
What is the potential for improving the clinical relevance of APSD data for inhalers?
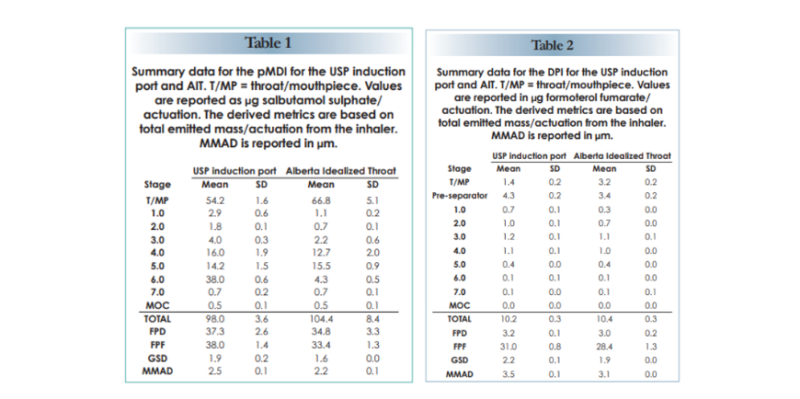
The tables below show comparative data for a commercial pMDI (active ingredient salbutamol sulphate) and DPI (active ingredient formoterol fumarate). Data were collected using the Next Generation Impactor equipped with either the AIT or USP/Ph. Eur. induction port.
In both instances, the AIT retains a higher mass than the USP/Ph. Eur. induction port. But that’s not all. The use of the AIT shifts Mass Median Aerodynamic Diameter (MMAD) to a finer size, indicating the preferential capture of larger particles. All of the APSD metrics are affected: Fine Particle Dose (FPD) and Fine Particle Fraction (FPF) are lower, and Geometric Standard Deviation (GSD) narrows.
The bottom line: using the AIT improves the clinical relevance of the measurement with respect to both the quantity and APSD of the dose that will reach the lung.
These are important gains for accurate assessment of the likely success of drug deposition.
And what about nasal drug products?
As discussed in earlier blogs, for nasal drug products cascade impaction is used to assess the risk of off-target delivery rather than the likelihood of success. However, the same principles apply. Using a more clinically representative model of the nose results in a more relevant APSD measurement for assessing the risk of pulmonary deposition.
The Alberta Idealised Nasal Inlet (AINI) is specifically designed for this purpose (and for broader studies of nasal deposition).
Here’s a test set-up incorporating the AINI for clinically representative APSD measurement for a nasal drug product.
Also shown is a breathing simulator, a second critical element of a test set-up for more clinically representative measurement for all OINDPs. Look out for the second blog in this series which covers their use.
In the meantime, if you want to find out more about clinically representative test set-ups for all types of OINDP then visit our website. Alternatively, if the new PSGs and realistic APSD are your focus a the we recently published a Q&A on this topic.