Contact Us
Contact Us

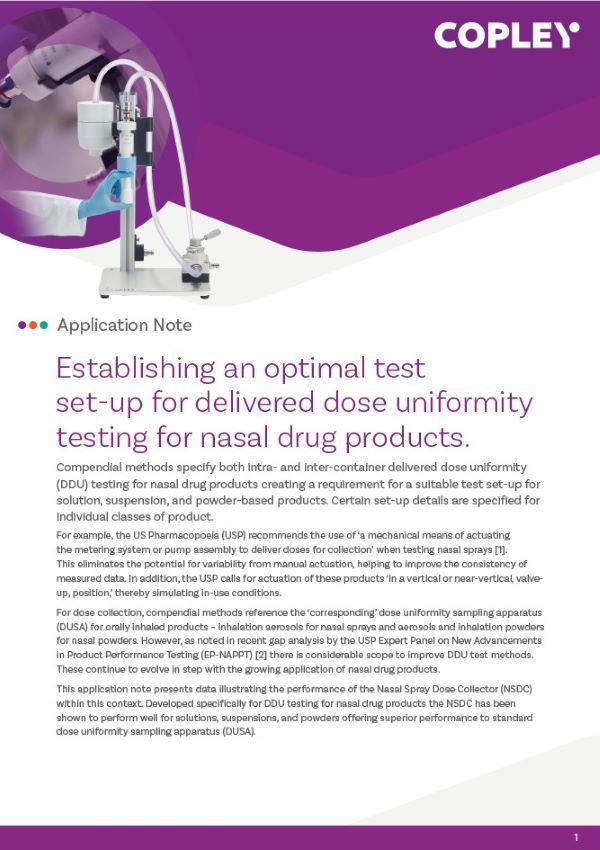
NGI Dissolution Cup and Membrane Holder
Based on a concept developed by Professor Jason McConville at the College of Pharmacy, University of Texas, USA the NGI Dissolution Cup and Membrane Holder incorporates a modification of the standard NGI collection cup. It allows size-fractionated particles from an aerosol cloud to be collected and then tested in a conventional tablet dissolution tester.
Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out More