Contact Us
Contact Us
Please select one of the DISi Series dissolution testers for further product information
Easy-clean Viton® membrane-sealed zero-evaporation lids included as standard
Intuitive touchscreen control with icon-based menu structure simplifies operation
Robust metal case with advanced corrosion protective coating
PT100 temperature probe monitors bath and vessel temperature with electronic calibration feature
Independent digital heater/circulator maintains a constant temperature and minimises vibration
Easily removable leak-proof water bath with convenient drain tap eases bath water emptying
All vessels are serial-numbered as standard
Convenient screw-in basket/paddles enable easy method change in seconds
Maximum fill line indicator
‘Easy-Centre’ centring system for precise vessel positioning
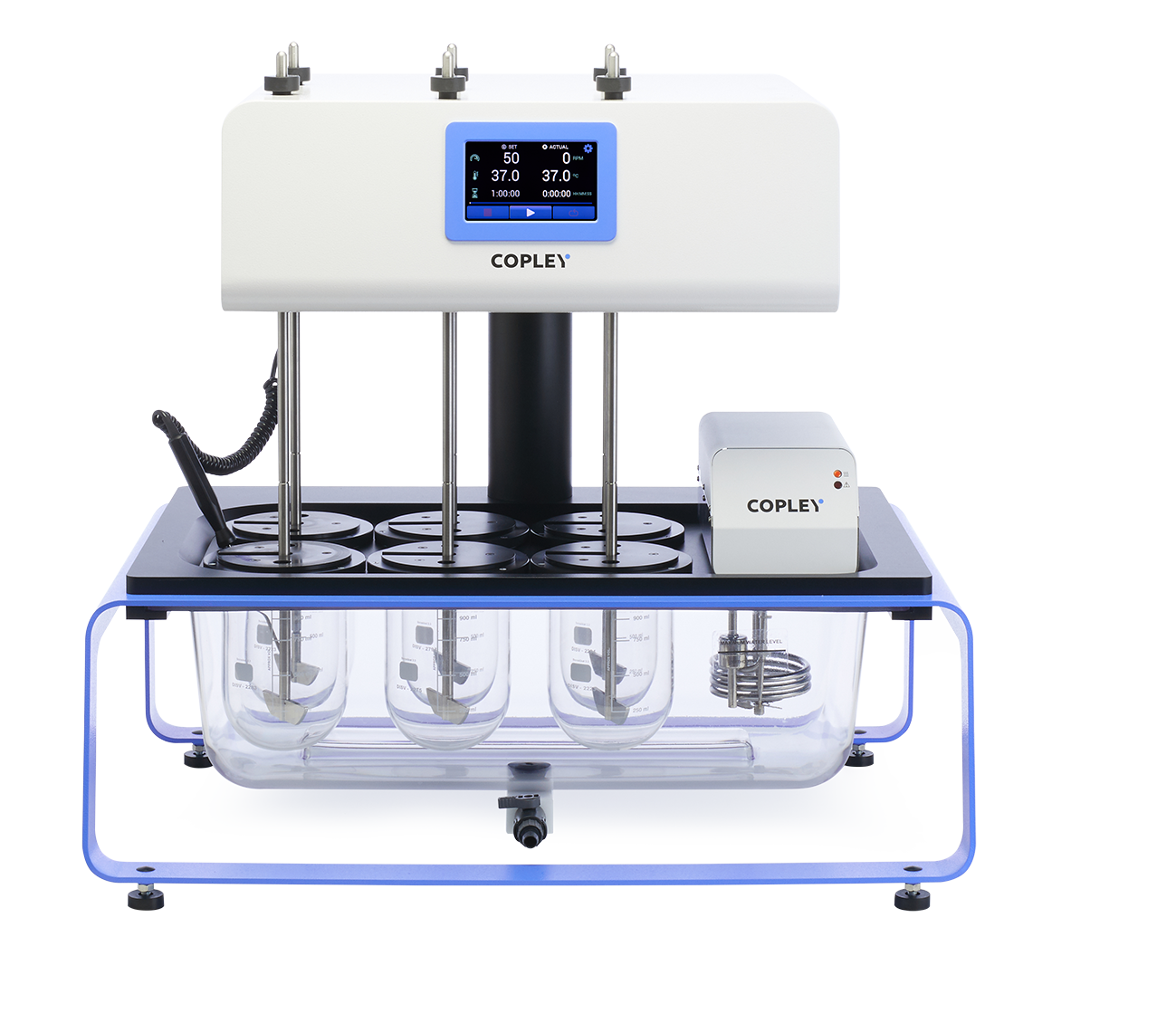
DIS 600i
With bench space a premium in many laboratories, the DIS 600i is one of the most compact dissolution test for tablets available on the market today.
Ideal for use in both R&D and QC environments, the DIS 600i is equipped with precision ground shafts that will accept any of the baskets, paddles or rotating cylinders described in the Ph. Eur., USP and associated Pharmacopoeias.
Catalogue Number: |
1336 |
Pharmacopoeial Compliance: |
Ph. Eur. 2.9.3 and 2.9.4 USP <711> and <724> |
Ph. Eur. and USP Test Methods Supported: |
1, 2, 5, 6 |
Number of Stirred Vessels: |
6 |
Heater Type: |
Low vibration independent external digital heater/circulator |
Unit Dimensions (w x d x h): |
728 x 495 x 689 mm (unit) |

Please select one of the DISi Series dissolution testers for further product information

Easy-clean Viton® membrane-sealed low evaporation lids included as standard
Intuitive touchscreen control with icon-based menu structure simplifies operation
Robust metal case with advanced corrosion protective coating
PT100 temperature probe monitors bath and vessel temperature with electronic calibration feature
Independent digital heater/circulator maintains a constant temperature and minimises vibration
‘Easy-Centre’ centring system for precise vessel positioning
Maximum fill line indicator
Convenient screw-in basket/paddles enable easy method change in seconds
All vessels are serial-numbered as standard
Easily removable leak-proof water bath with convenient drain tap eases bath water emptying
DIS 800i
Maximising visibility and access to the critical sampling area above the bath, the DIS 800i represents the very latest in tablet testing technology.
Ideal for use in both R&D and QC environments, the DIS 800i is equipped with precision ground shafts that will accept any of the baskets, paddles or rotating cylinders described in the Ph. Eur., USP and associated Pharmacopoeias.
Catalogue Number: |
1338 |
Pharmacopoeial Compliance: |
Ph. Eur. 2.9.3 and 2.9.4 USP <711> and <724> |
Eur. Ph & USP Test Methods Supported: |
|
Number of Stirred Vessels: |
8 |
Heater Type: |
Low vibration independent external digital heater/circulator |
Unit Dimensions (w x d x h): |
728 x 495 x 689 mm (without heater) 260 x 330 x 150 mm (heater) |

Key DISi Series Highlights
DISi Series dissolution testers are Ph. Eur. and USP compliant
Integrated, precision temperature control and measurement
Intuitive touchscreen control to simplify operation
Single-point electronic temperature calibration
Six and eight test station unit configurations available
Extensive data reporting output options: RS 232, USB A and USB B
Wide speed range to accommodate broad scope of methods
Option to automate and remotely control DISi Series systems
DISi Dissolution Tester Series

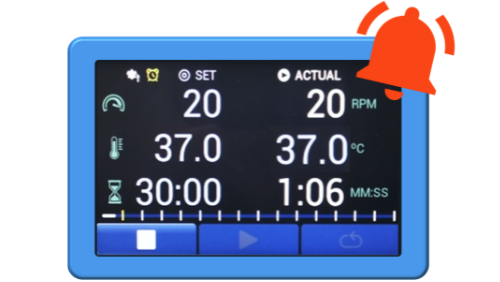
DISi Sampling Alerts
- Enter timepoints for sampling
- Begin testing
- Receive warning alert prior to sample timepoint
- Get alerted at the required sample timepoint
- Never miss a sample!


DISi Compliance & Maintenance
- Certificate of compliance to Ph. Eur./USP provided as standard
- Comprehensive IQ/OQ/PQ documentation packages and toolkits available
- Passcode-protected single-point electronic temperature calibration
- Latest temperature calibration information stored and available to export/print


DISi Series Calibration Tools
- Calibration of the DISi Series temperature probe is simple, through the use of an electronic calibration key and pin-protected calibration menu designed to guide users through the process without fuss. The latest temperature probe calibration information is stored and available to print/export when convenient to the user.
- To calibrate all other aspects of the DISi Series, our complete range of calibration and qualification tools can be used to safeguard compliance with the appropriate pharmacopoieal and regulatory guidance.
Special Applications
Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out More










