5 August 2008: Nottingham, UK: Copley Scientific has officially launched CITDAS V3.00 (Copley Inhaler Testing Data Analysis Software), a new version of the company’s well-established, fully validated software package for the analysis of cascade impaction data. Cascade impaction is a technique specified for testing all inhaled products and is essential in the pharmaceutical industry.
CITDAS accepts results from all commonly used impactors and generates all the data analysis specified by the US and European Pharmacopoeias for inhaler testing. The latest version of the software incorporates a host of new features developed in response to customer feedback, and the changing requirements of the regulatory authorities.
CITDAS V3.00 includes calibration data for the Next Generation Pharmaceutical Impactor (NGI) in the flow range 15 – 30 L/min, broadening its applicability, particularly in the area of nebuliser testing. Enhanced options for data input, output and manipulation, as well as the ability to specify stage groupings, provide added flexibility. CSV files can now be imported, as well as exported, eliminating the need to manually input data, from HPLC for example. Any raw input data appears on printouts for better traceability, and data resolution can be changed as required.
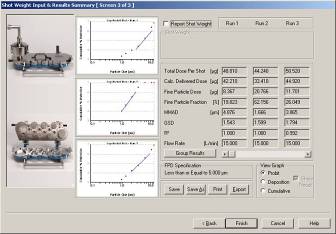
CAPTION: CITDAS V3.00 software screen shot
About Copley Scientific
Copley Scientific is recognised as the world’s leading manufacturer of inhaler test equipment and is a major supplier of test equipment for pharmaceutical solid dosage forms, including tablet dissolution, disintegration, friability, hardness and powder testers.
The company has offices in the UK and Switzerland and a partnership with aerosol particle science experts MSP Corporation in the US. Copley’s broad range of testing products for metered-dose inhalers, dry powder inhalers, nebulizers and nasal sprays are supplied and supported worldwide through close relationships with specialist distributors. Serving the pharmaceutical and associated industries, Copley offers an extensive range of equipment for research, production, clinical trials and quality control, as well as full validation and aftersales service, providing a single source for products that meet individual needs.







