The right software can speed you from raw cascade impactor data to aerodynamic particle size distribution (APSD) metrics and actionable insights in seconds…
Cascade impactor measurements produce a lot of data. Chemical analysis of each recovered fraction results in a stream of assay data for every APSD measurement. Crunching through that data quickly and consistently, to generate critical product metrics, is crucial for efficient inhaler testing.
Software is the solution, but flexibility and usability are key. Every product has a unique cascade impactor test set-up and every lab its own assay protocols.
Inhalytix speeds you from test method definition and data entry, through data analysis to the information you need
We’ve developed Inhalytix to meet these diverse requirements, to support the preparation, execution and analysis of every cascade impactor measurement. Inhalytix allows simple, manual data input from every type of HPLC system and can be cloud-, PC- or server-based, depending on your requirements.
Take a closer look at how Inhlaytix accelerates and improves APSD data analysis by:
1. Handling products with multiple actives
Dual and triple active metered dose inhalers (MDIs) and dry powder inhalers (DPIs) dominate today’s commercial landscape with respect to newly approved therapies for asthma and COPD. Such products hold promise for better adherence and synergistic therapeutic effect but bring considerable complexity. The scope for drug interaction within the device and unequal dispersion necessitates rigorous APSD analysis for each active. Achieving and demonstrating bioequivalence is especially demanding.
Inhalytix handles multi-active products with ease. Simply add each active of interest as you define the test methods and then analyse to report drug-specific APSD metrics for each one. Whether in development or QC, this capability is crucial as products, and by extension analyses, become more complex.
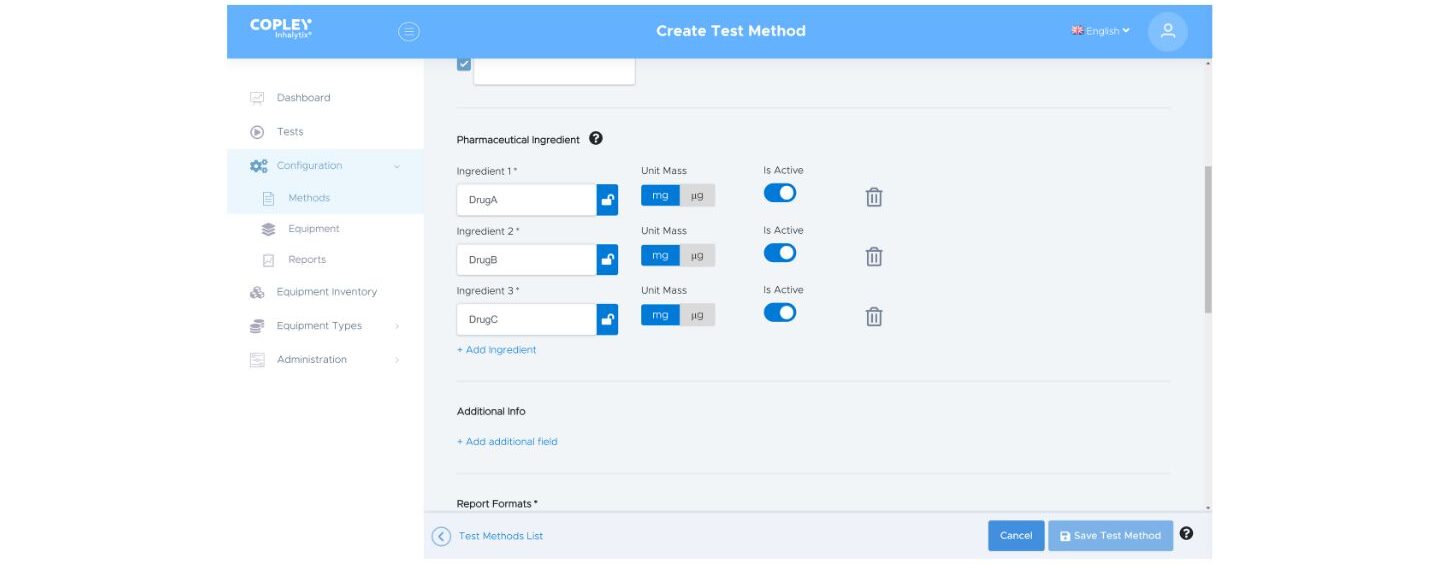
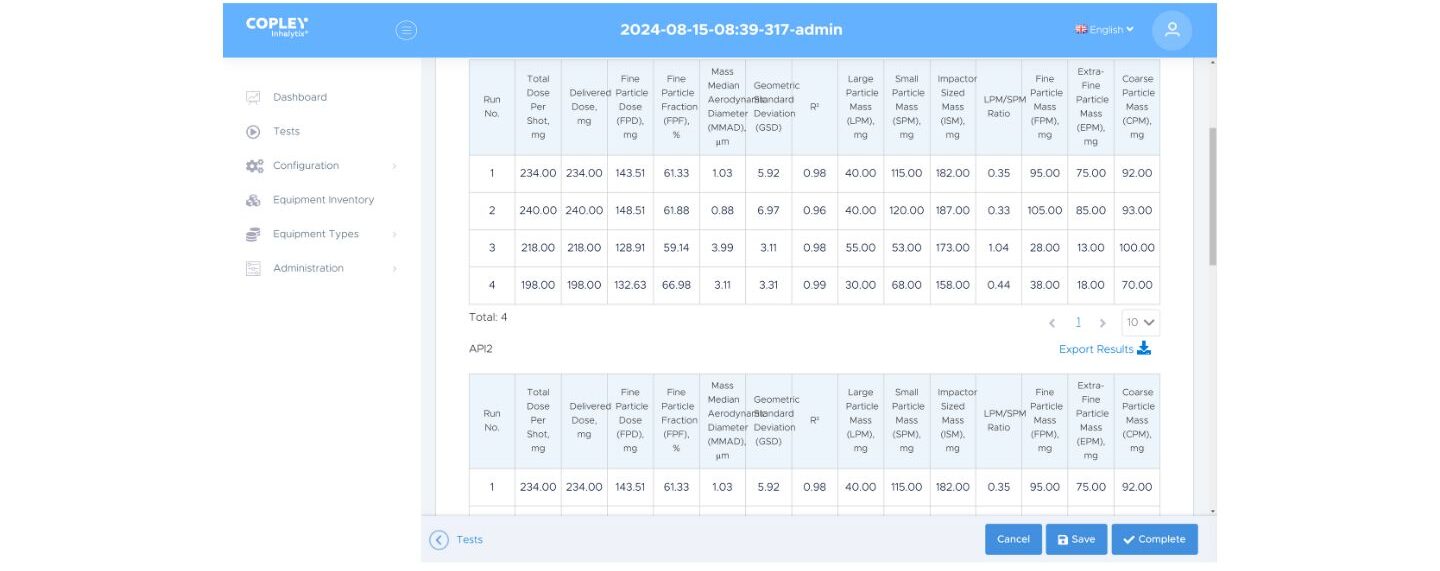
Simply ‘Add ingredient’ to define a test method for multiple actives (top), Inhalytix will then report the metrics of interest for each individual drug (bottom)
2. Delivering fully customisable reports
Depending on the product and where you’re working – in R&D or quality control – the APSD metrics of most relevance may vary. You may want to focus on Mass Median Aerodynamic Diameter (MMAD) and Impactor Sized Mass (ISM) or on Fine Particle Dose (FPD). You may be interested in abbreviated measurement and the ratio of Large Particle Mass to Small Particle Mass (LPM/SPM). Comparison with a reference, for batch-to-batch QC or to assess bioequivalence may be highly desirable.
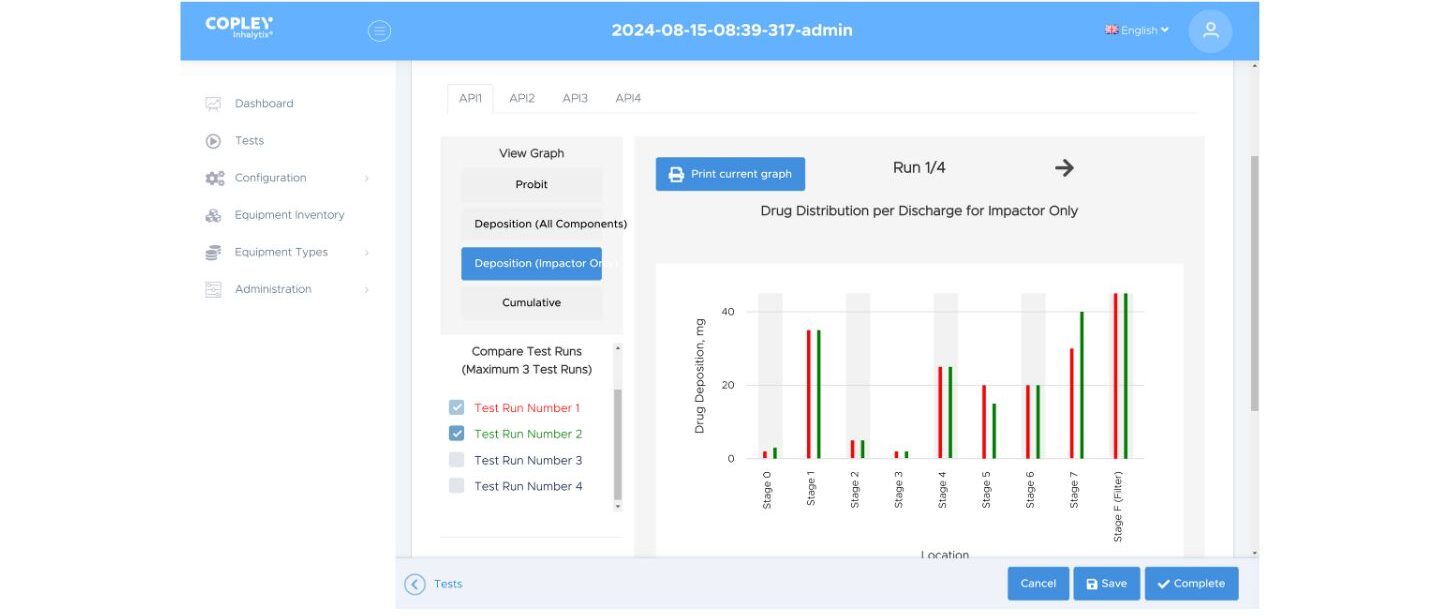
With Inhalytix you have the full range of APSD metrics at your fingertips. Simply choose those of interest and pull them into a report format that works for you. Link the result with a defined method to ensure consistent data presentation, every time.
3. Keeping track of equipment
Busy test labs rapidly acquire a wide range of inhaler testing equipment. Examples range from different types of cascade impactors, and conversion kits (for the ACI), to different induction ports and pre-separators. Customised equipment may also be part of the mix.
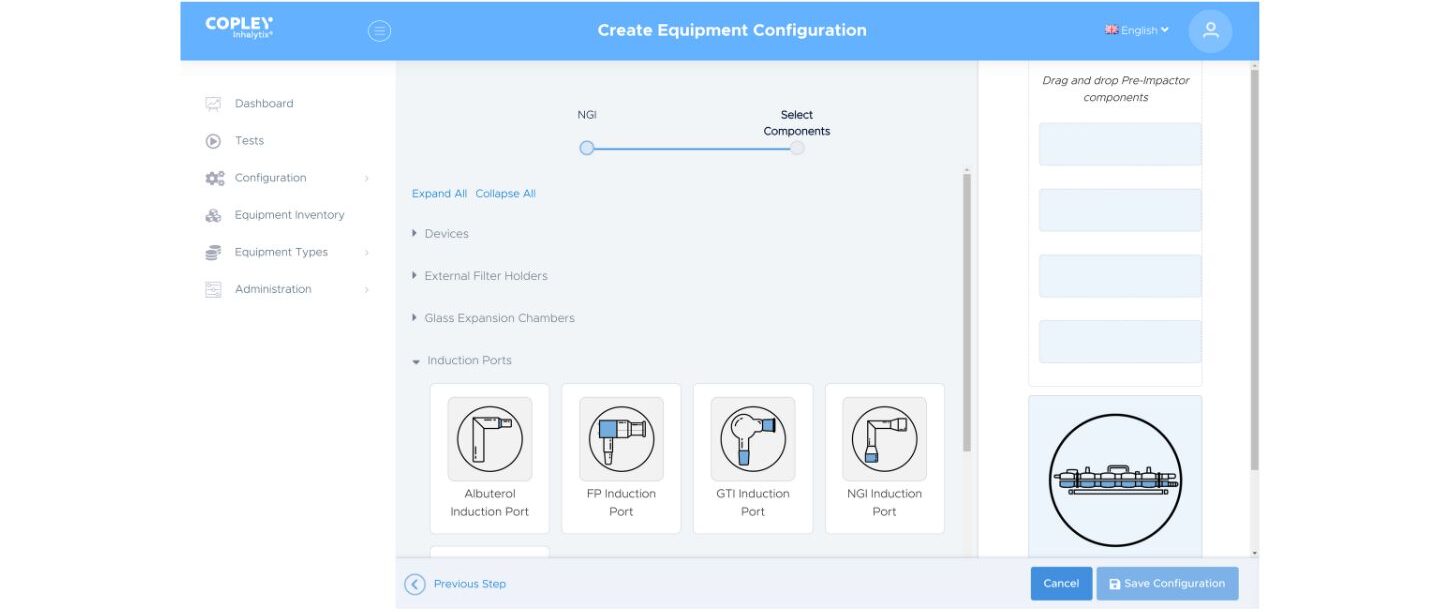
With Inhalytix its simple to keep track of all these assets via an on-screen equipment inventory. By clearly logging and displaying all relevant apparatus this inventory makes definition of a test set-up straight forward. Linking specific pieces of equipment with defined test methods ensures rigorous consistency. Furthermore, the equipment inventory can be updated with critical information such as stage mensuration data if required.
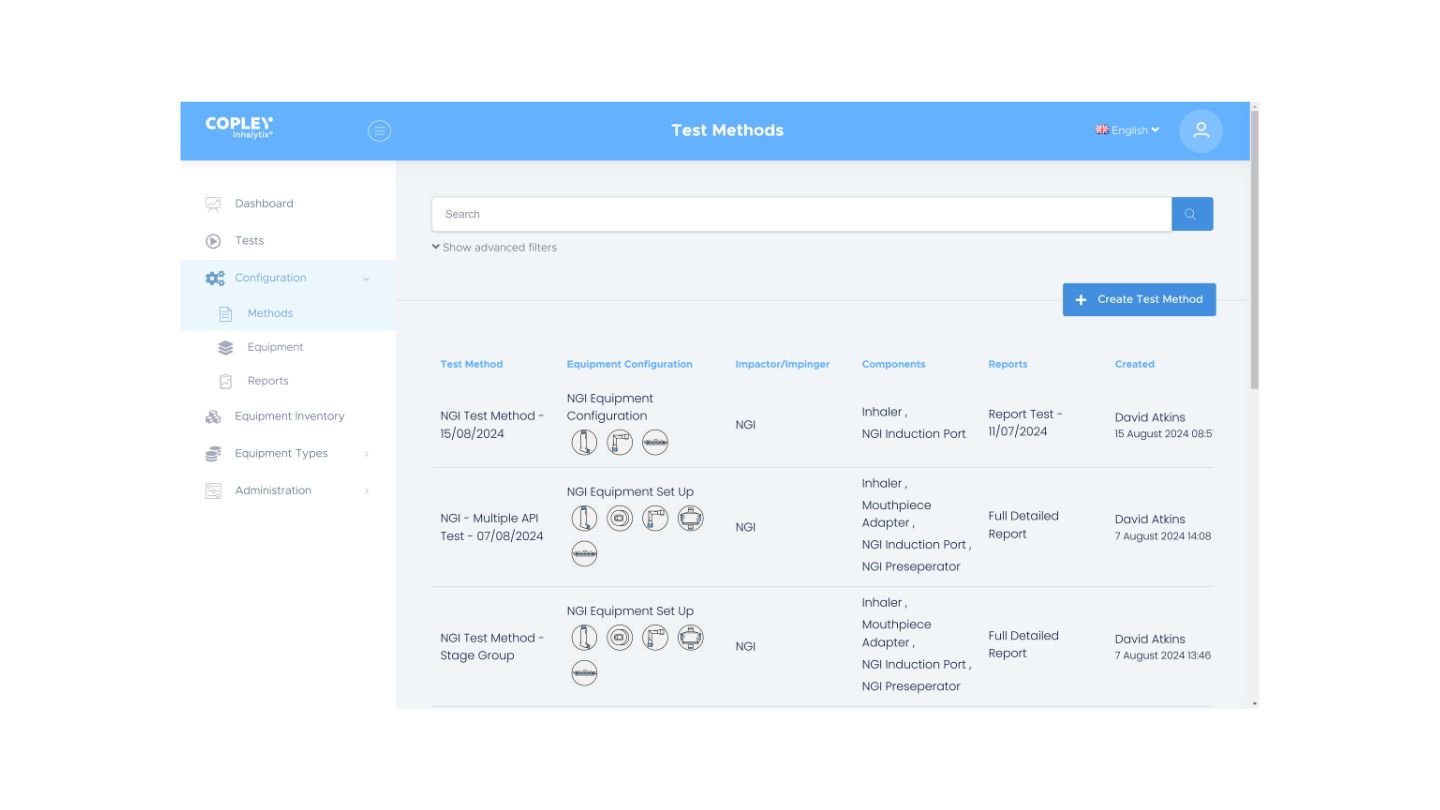
4. Streamlining the demonstration of compliance
The data security and regulatory compliance needs of the pharmaceutical industry are well-known but can create a considerable burden for analysts. We’ve built Inhalytix to lighten this load.
Inhalytix is:
- Compliant with all major regulatory requirements and pharmacopoeial specifications: US (FDA/USP), European (EMA/Ph. Eur.), Chinese (CFDA/ChP) and Japanese (PMDA/JP)
- 21 CFR Part 11 ready
- Fully validated, with one-click in situ automatic validation
The result is secure, audit-ready inhaler testing data with minimal effort.
5. Improving lab management
All labs are under increasing pressure to do more with less by optimising their use of all resources. Workflows that support analyst productivity, clarity around equipment usage and requirements, and efficient data reporting all contribute to such efforts.
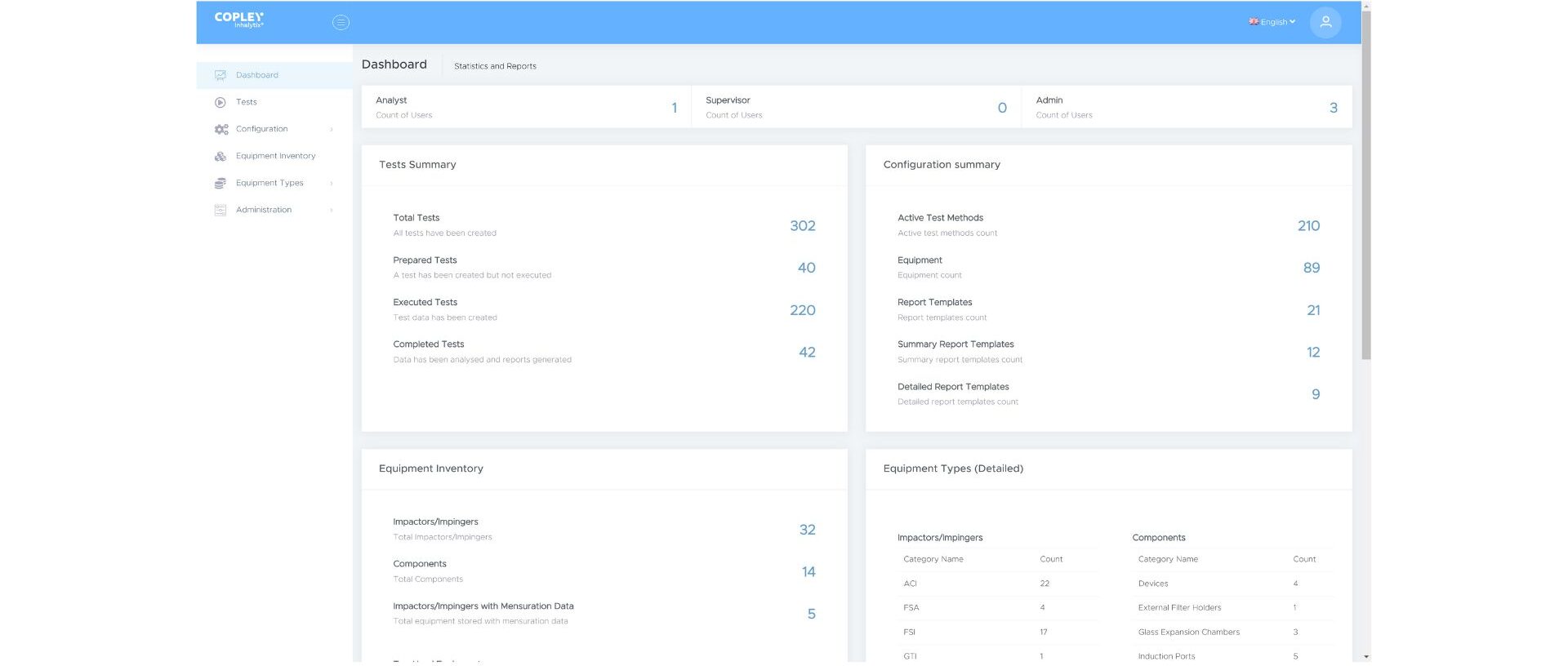
Customers routinely highlight the benefits of the Inhalytix dashboard within this context. Upon login the dashboard displays usage metrics in real-time. It provides details of the latest tests and summarises all available test, equipment and report configurations. All the information you need, clearly displayed, at your fingertips.
The bottom line
Whether recording test status, keeping track of equipment, guiding analyst workflow or improving information presentation, Inhalytix can transform cascade impactor data analysis efficiency. If you’re new to Inhalytix then this video provides a great introduction, alternatively if you’d like a demo then just ask.








