Controlling temperature, relative humidity (RH), and electrostatics is critical when it comes to inhaler testing. Find out why…
When inhaler testing data, notably cascade impaction measurements, exhibit unacceptable levels of variability the answer may be better flow control. Or perhaps automation, if the issue is operator dependent. But what about the test environment – could that be the problem?
Have you ever considered how temperature and RH change through your lab, through the day, and across the seasons, and how that impacts measurements? And what about the efficacy and consistency of your electrostatics mitigation strategies?
In inhaler testing, temperature, RH and electrostatic effects in the localised environment are all potentially significant sources of variability. And too-often overlooked. This blog is all about environmental control, why it’s important and how to achieve it.
How does the test environment influence inhaler test data?
This fundamental question that has two discrete answers: (1) by influencing dose delivery and (2) by influencing cascade impactor performance.
For orally inhaled products (OIPs) dose delivery is a dynamic process. Actuation releases an aerosolised dose to the patient, or into the test apparatus, but the characteristics of that dose are not fixed. Factors that impact these characteristics impact all forms of OIP testing.
In addition, variability from multiple sources can compromise cascade impactor performance and by extension aerodynamic particle size distribution (APSD) measurements.
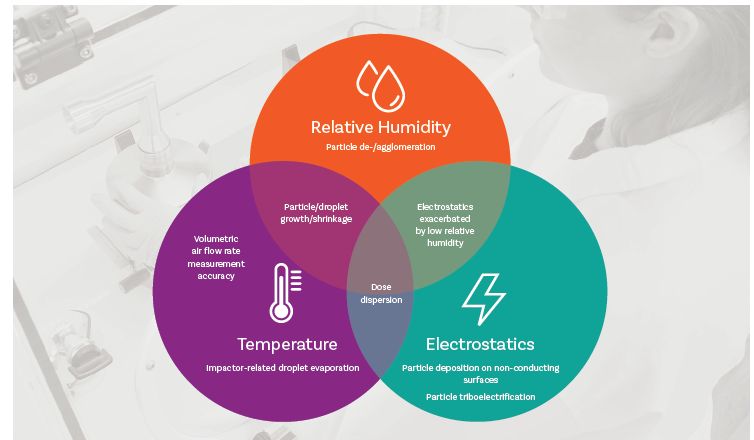
Variability in the test environment can impact inhaler testing via mechanisms associated with both issues, as summarised below.
Variability in temperature, RH and electrostatic effects in the localised environment can influence the characteristics of the delivered dose and/or performance of the test set-up
Temperature
Temperature influences dose dispersion directly by affecting vaporisation rate, notably of propellants. It can also further affect atomisation behaviour because of the temperature dependence of properties such as viscosity and surface tension.
Turning to test equipment there is a temperature-dependent risk of droplet evaporation within the impactor that can skew APSD measurements. Compendial requirements to cool the Next Generation Impactor (NGI) to 5oC when testing nebulisers reflect this issuei,ii, likewise current best practice for testing soft mist inhalers which similarly recommends the use of impactor coolingiii. In addition, temperature variability can hamper efforts to accurately measure and control flow rate, as required for consistent impactor separation performance.
RH
RH influences the particle/droplet formation processes that occur during dose delivery, thereby impacting the characteristics of the delivered dose. This is true for both liquid and powder formulations, with powder deagglomeration notably susceptible to moisture levels.
Electrostatics
The energetic nature of dose dispersion can give rise to tribolectrification, the generation of electrostatic charge by contact and separation. Inducing electrostatic effects during testing is therefore relatively easy. This is a complex area to study with rigour, but electrostatic effects are known to impact dose dispersion and deposition behaviour in an impactor. Variability in electrostatic generation and dissipation is therefore potentially problematic.
While each of these factors individually influences test data, interplay between them further complicates variability effects. For example, temperature and RH in combination influence the atomisation processes that define the characteristics of dispersed liquid formulations. And RH has a pronounced effect on electrostatics. Because water is highly conductive, electrostatics effects are less pronounced at higher RH levels with 40 – 60% ensuring significant leakage to ground.
Strategies for mitigating environmental variability
NGI cooling
Compendial requirements to chill the NGI for nebuliser testing calls for refrigeration. One option is to chill the impactor in a lab refrigerator (typically for around 90 mins) and then complete testing before any appreciable warm-up. An alternative is to use a unit such as the NGI Cooler™, designed specifically to address this issue. It offers energy efficient temperature control across the range 0 – 10oC.
General test environment control
For more comprehensive control of the inhaler test environment, thoughts naturally turn to air conditioning (A/C – temperature) or full climate control (temperature + RH) of the lab. Unfortunately, both the upfront and running costs of such systems are prohibitive for many. This is especially true in geographies that impose a heavy working load on the system, and/or if energy security, reliability and cost are problematic.
Furthermore, even if A/C or climate control systems are already in place it is worth checking how effectively they maintain consistent conditions at the workstation. Heat release by in-use equipment, drafts, windows and vent positioning can all have a potentially problematic localised impact.
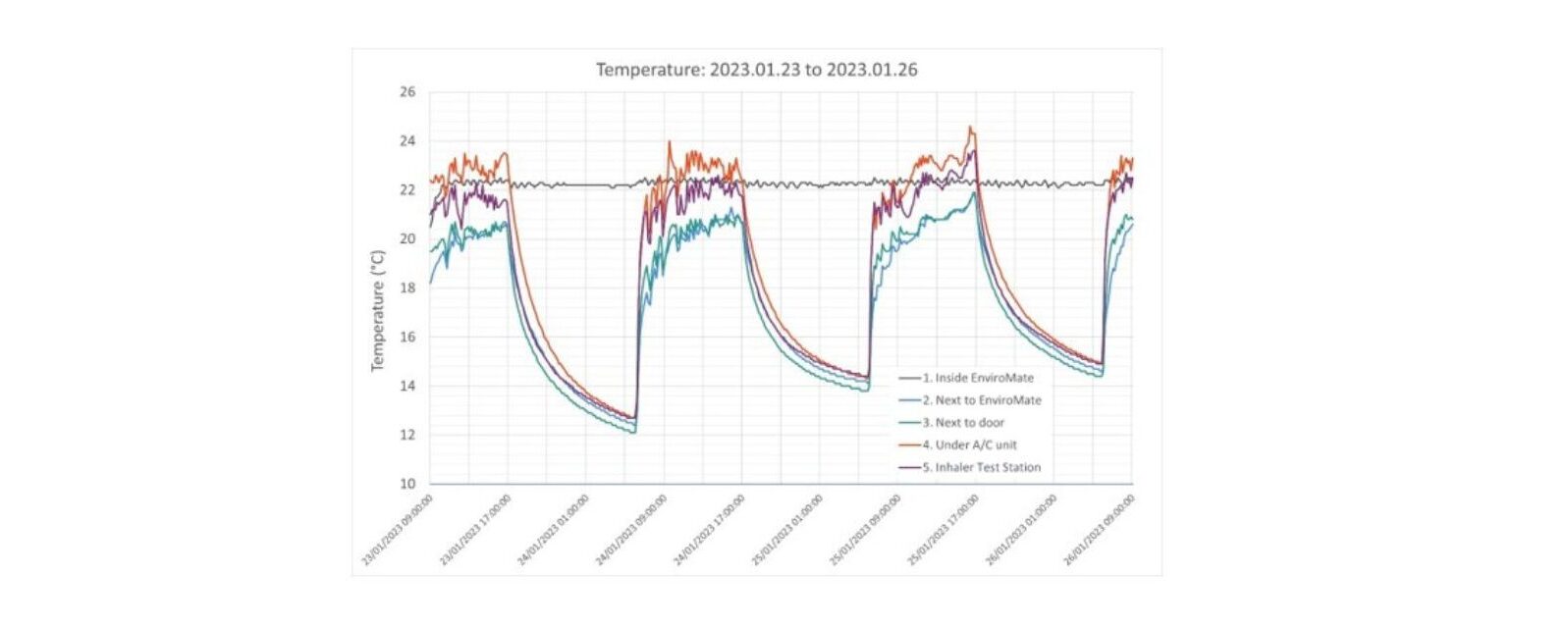
The alternative to relying on lab-wide systems is to use individual climate-controlled chambers. These can be highly effective, offering flexibility and uniform control in combination with relatively low upfront and running costs. For example, EnviroMate™ our benchtop unit designed specifically for inhaler testing, provides accurate temperature (+/-2oC) and RH (+/-5%) control throughout. It also incorporates an integrated anti-static system to minimise electrostatic effects.
Focusing on electrostatics
Robust temperature and RH control is an important first step towards minimising electrostatic effects, but additional steps may be helpful/essential. Useful strategies include:
- Grounding (earthing) both equipment and operators
- Restricting glove use and the type of glove worn
- Selecting the right protective lab equipment, anti-static shoes being a prime example
- Deploying electrostatic eliminators and guns, and ionisation bars
Handheld static meters are useful for checking the effectiveness of any electrostatic mitigation steps taken.
To learn more about the impact and benefits of environmental control, read our whitepaper on the topic. In it you’ll find information on how much your testing environment might be varying, and crucially, some insight into what that means for test data. By improving data integrity and reducing erroneous out-of-specification/out of trend results, better environmental control can deliver tangible dividends from R&D through to QC. Definitely worth considering, to ensure the highest levels of data integrity.
i USP <1601> Products for nebulization – Characterization tests
ii Ph. Eur. 2.9.44 Preparations for nebulisation: characterisation
iii J. Mitchell et al ‘Good Practices for the Laboratory Performance Testing of Aqueous Oral Inhaled Products (OIPs): an Assessment from the International Pharmaceutical Aerosol Consortium on Regulation and Science. AAPS PharmSciTech (2023) 24:73