OINDP testing has its own language with terms that can challenge newcomers. Here’s our round-up of some of the most common phrases.
All branches of science have their own language and inhaled drug delivery is no different. Seasoned OINDP analysts routinely use terms that are unique to the field or ‘borrowed’ and applied specifically. Newcomers fall rapidly into using the same terms, often in the form of acronyms, sometimes without fully grasping their specific meaning or implications.
In this blog we’re going to take a close look at five essential terms that every inhaler testing analyst should know. Our aim is to offer something new or significant context that even seasoned professionals might find surprising. Whether you’re new to the world of inhaled drug product testing or looking to sharpen your expertise, it’s worth revisiting the fundamentals.
APSD – Aerodynamic Particle Size Distribution
What is it? Aerodynamic particle size quantifies particle diameter on the basis of how particles behave in a moving airstream. Orally inhaled product doses rarely if ever contain particles that are all identical in size, so we report Aerodynamic Particle Size Distribution (APSD) rather than a single value. APSD shows how a sample is distributed with respect to size on the basis of mass, i.e. what mass of particles lies within each fraction of the overall size range.
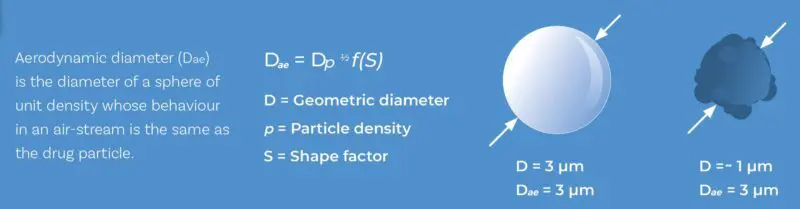
Aerodynamic particle size or diameter is influenced by both size and shape but crucially, it defines how a particle behaves in air, making it highly relevant to OINDPs.
Why it matters? APSD is a performance-defining characteristic for all orally inhaled products (OIPs) as we’ve discussed in an earlier blog.
What to keep in mind: Even though APSD is a familiar term, a few important reminders are worth highlighting:
- ‘Aerodynamic’ is critical. APSD is not the same as Geometric Particle Size Distribution (GPSD) (typically measured by laser diffraction) or PSD measured in any other way. Particle sizing techniques generate data from measurements of different aspects of particle behaviour. We choose APSD because of its relevance to how particles behave in a flowing air stream, and by extension how they are drawn into the body on an incoming breath. Which brings us to…
- Aerodynamics within the cascade impactor determine measurement precision. Our ability to accurately measure APSD by cascade impaction is dependent on precise control of the aerodynamic regime within the impactor. There are three aspects to this:
- A calibrated cascade impactor manufactured to precise tolerances.
- Routine maintenance to preserve those original dimensions.
- Accurate control of air flow during every measurement.
- APSD measurement and dissolution are linked for OIPs. If we want to understand how inhaled particles dissolve, then the starting point is the respirable fraction. While general correlations between particle size and dissolution are well known, what needs more research is an understanding of how the respirable fraction dissolves under conditions that are representative of the lung. Cascade impaction provides the best starting point for isolating particles in this range.
IVIVC – In Vitro-In Vivo Correlation
What is it? IVIVC describes the relationship between results measured in the lab and behaviour in the body.
Why it matters? For all forms of pharmaceutical product development, strong IVIVCs allow for more meaningful in vitro testing that reduce reliance on clinical trials and can accelerate product development.
What to keep in mind: For OINDPs, establishing robust IVIVCs remains a major challenge, as we’ve highlighted in earlier blogs.
Why? Physiology is a primary reason; the route to systemic circulation is long and complex. For example, the lung has many bifurcating pathways of progressively diminishing diameter to navigate; fluid levels are low. The nasal cavity has mucociliary clearance, a highly effective defence mechanism.
Historically with OINDPs, the goal has been localised action so the post-deposition fate of inhaled particles has been a safety concern rather than an issue of efficacy.
But this is changing.
With the development of vaccines and systemic therapies, a more complete understanding of the in vivo pathway is becoming more critical. This shift is driving:
- More physiologically relevant in vitro methods
- Advances in physiologically based pharmacokinetic modelling (PBPK)
The closer we can get to robust IVIVCs for OINDPs, the more we can experiment rapidly and cost-effectively in the lab.
Even with localised products there is room for improvement. There is potential to tune the sub-5 µm dose to achieve more effective deposition and efficacy in different areas of the lung. If we can develop the in vivo understanding to do so. And crucially, for generic products, the potential to demonstrate bioequivalence with minimal in vivo testing.
FPD – Fine Particle Dose
What is it? The mass of respirable particles in a dose, typically those with an aerodynamic particle size of less than 5 µm.
Why it matters? 5 µm is typically considered the upper particle size limit for deposition in the lung which is why FPD is one of the most common metrics reported for OIPs.
What to keep in mind: Increase FPD and you’re heading towards more efficient drug delivery to the lung, and by extension better clinical efficacy. Confirm that FPDs are equivalent to confirm batch-to-batch comparability. Right?
Well not always. It’s easy to assume FPD tells the whole story, but it doesn’t. The APSD of OIPs can differ – sample to sample, batch to batch and product to product – in two ways:
1. There may be changes in the amount of fines and/or or coarse material, i.e. the shape of the particle size distribution OR
2. An overall change in mass.

APSDs can differ with respect to particle size (left and centre) or mass collected (left and right)
Since FPD defines the respirable mass it’s excellent at picking up this last type of change. But not so much a change in particle size distribution that occurs in the sub 5 µm region. One product could have substantially more fines than another and still have the same FPD (see centre plot above). In which case FPD equivalence might not equate with bioequivalence.
In summary, FPD is enormously helpful, but not, single-handedly, capable of capturing changes you may want to be aware of. For full insight, it should be interpreted alongside other APSD metrics.
EDA – Efficient Data Analysis
What is it? EDA is a method of using all the data that flows from a cascade impaction measurement in a more efficient way, by grouping stages and reporting fewer fractions.
Why it matters? It streamlines data analysis and at the same time can improve precision.
What to keep in mind: Typically, grouping data reduces resolution. EDA doesn’t do this. Rather it minimises some of the potential, but unavoidable, inaccuracies and limitations associated with the analysis of cascade impaction data.
The APSD of a typical OIP results in less drug deposition on the first and last stages of a cascade impactor, relative to the central stages. Depending on the sensitivity of the assay equipment used, this can make detection challenging and lead to null results or significant errors. Increasing the number of actuations addresses this issue but increases experiment time, consumes more of the drug product, potentially increases variability and can lead to overloading of the centre stages. It’s a crucial balancing act to determine the number of actuations required to achieve detection limits, without causing an overload.
If we group stages, as with EDA, the mass of product in each group is larger, relative to the mass on each individual stage. This therefore makes it possible to produce accurate metrics of interest while minimising actuations. That said, we need to be sure that the use of EDA metrics doesn’t obscure critical changes in a given particle size range, as we have seen FPD can do with sub-5 µm particles.
In summary, EDA can streamline data analysis and enable the use of Abbreviated Impactor Measurement (AIM), cutting analysis times. At the same time, it can improve data accuracy and value, making it easier to effectively compare products. This win-win is both unusual and extremely valuable.
Critical Flow
What is it? Critical flow happens when a gas is moving through a narrow opening so fast that it hits the speed of sound (at the temperature/pressure of the surrounding environment). At this point, it doesn’t matter what’s happening downstream of the opening – the flow rate stays the same. This is called “choked flow,” and it means the amount of air going through is controlled only by the pressure on the upstream side. That means no matter what happens downstream (e.g. changes in vacuum pressure), the flow rate remains stable.
This critical point is reached once pressure downstream of an orifice or valve falls to ≤ 0.5 of the upstream pressure.
Why it matters? In dry powder inhaler (DPI) testing, achieving stable flow is especially important because delivery and dispersion performance are closely tied to how air flows through the device. As a result:
- Critical flow is part of the compendial requirements for both delivered dose uniformity (DDU) testing and aerodynamic particle size distribution (APSD) measurement for DPIs.
- A critical flow controller is a standard and necessary part of any DPI testing setup.
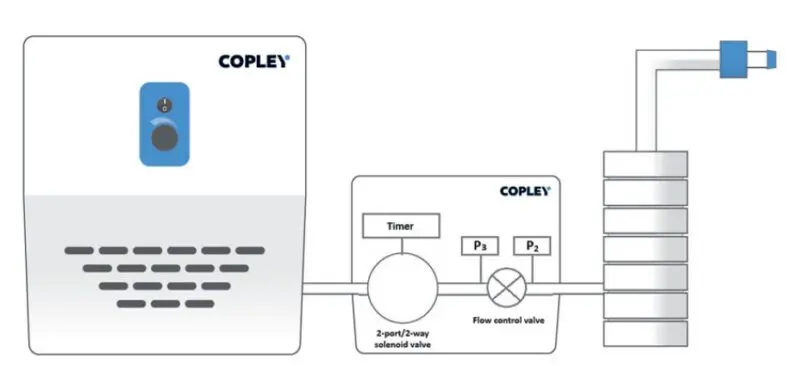
Schematic of an APSD measurement system for DPIs showing a flow controller between the cascade impactor (right) and vacuum pump (left)
What to keep in mind: We can see the practical benefits of achieving critical flow conditions by looking at a typical set-up for measuring the APSD of DPIs (see above). In this diagram pressure drop is running from right to left, towards the vacuum pump. Once the downstream pressure (P3) is less than half of the upstream pressure (P2), flow becomes independent of the vacuum side. That means:
- Any fluctuations on the vacuum pump side have no impact on mass flow rate
- The device is tested under consistent, repeatable conditions
In summary, achieving critical flow helps remove a variable from the system, gives you better control over your inhaler testing set-up and ultimately cleaner and more meaningful data.
Final Thoughts
Understanding key terminology is more than a box-ticking exercise; it’s about ensuring meaningful, accurate and efficient testing. As the global leader in inhaler testing equipment and expertise, we’re here to help you go beyond compliance to real confidence.
Make sure you subscribe to our blog for regular updates on OINDP testing trends, techniques, and expert advice straight to your inbox.








