Find out why cascade impaction and laser diffraction report different particle size data, and the significance for nasal drug product testing.
Consider the table below which shows particle size distribution data for three spray dried caffeine formulations for intranasal drug delivery*. Look carefully at the centre and right-hand columns.
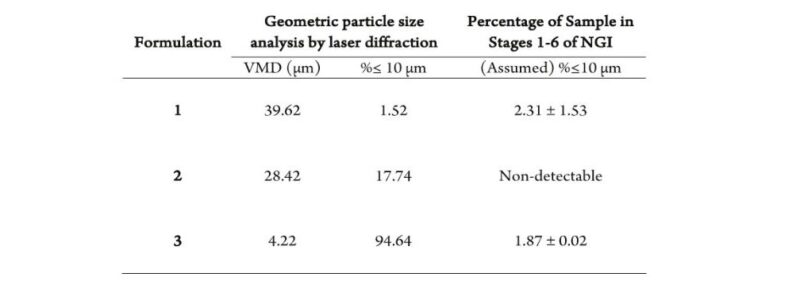
As we’ve discussed previously, in our blog on Safeguarding Nasal Drug Delivery, the sub-10 microns fraction is of critical concern for nasal drug products because it quantifies the risk of pulmonary deposition.
These data show clearly that the technique used to make that measurement is highly significant. There is no obvious correlation between the sub-10 microns fraction as measured by laser diffraction and by cascade impaction (with the Next Generation Impactor – NGI). The laser diffraction data indicate that the risk of pulmonary deposition is high; the cascade impaction data show it to be negligible.
In this blog we’re going to explore this perhaps startling result and the associated ramifications for inhaled drug product characterisation.
What does laser diffraction measure?
Laser diffraction systems measure the light scattering pattern produced when a collimated laser beam interacts with a sample of particles, in the case of nasal drug products, a cloud of aerosolised droplets or particles. They then back-calculate the size distribution of a particle population that would give rise to this scattering pattern by applying an optical model, either Fraunhofer or Lorenz-Mie. Both are fundamental laws of physics that describe the relationship between light scattering and particle size. This calculation is complex and iterative. It involves hypothesis of a particle size distribution, calculation of the associated light scattering pattern (using the chosen optical model), comparison with the measured data, adjustment of the proposed particle size distribution and so on.
The resulting metric is commonly referred to in the inhalation community as Geometric Particle Size Distribution (GPSD).
And why is it used for nasal drug products?
Laser diffraction is fast and efficient and comfortably covers the size range of interest for deposition in the nose. It allows researchers to measure the evolution of a nasal spray event in real time enabling them to see, for example, how quickly atomisation stabilises and to assess and compare droplet/particle size within the resulting fully developed phase. Such information is valuable for nasal drug product development and the technique is directly referenced by the regulatory guidance.
What does cascade impaction measure?
For a more detailed description of cascade impaction please refer back to the Cascade Impaction blog post, but in summary it measures an Aerodynamic Particle Size Distribution (APSD) for the drug substance by size fractionation of a dose on the basis of particle inertia and subsequent assay of the collected fractions.
Let’s unpick that statement with reference to laser diffraction.
Firstly, cascade impactors metrics are defined by aerodynamics, by the way in which particles move in air, specifically particle inertia. This is very different from laser diffraction which determines particle size on the basis of response to light.
Secondly, cascade impaction enables specificity. It is the concentration of drug in collected samples that is measured, not the entire formulation. Again, this sits in marked contrast to laser diffraction which sizes the formulation, with no capability to differentiate the drug.
And why is it used for nasal drug products?
For nasal drug products cascade impaction measures the amount of drug particles that, on account of their aerodynamic behaviour, are most likely to penetrate through the nasal cavity to the lung, thereby presenting a safety risk. Because the resulting APSD is for the drug itself, this is a robust, direct assessment with no assumption that the drug is evenly distributed across the dispersed dose.
Different and (un)equal
Armed with this background its easier to understand why the techniques deliver different information. We may think of particle size as a single variable, like particle density, but in fact, across industry many different particle size metrics are routinely reported, depending on the behaviour of interest.
However, this raises an important question. Can we convert GPSD to APSD and vice versa?
When using laser diffraction it’s not necessary to be aware of the mathematical processes that generate GPSD (other than choosing or inputting some suitable refractive index data) but it helps to understand the assumptions which underpin them which include that:
- Particles are spherical.
- Light only interacts with a single particle prior to detection.
It’s also worth noting that laser diffraction delivers volumetric particle size distribution data, in other words it determines the volume of sample that lies in each part of the size range.
These points are highly relevant to efforts towards conversion. Nasal drug products particles are not perfectly spherical and the extent to which they deviate from sphericity varies from product to product. Particle morphology also affects aerodynamic behaviour, but in a different way, that is more reflective of how it will change passage through the nasal cavity.
It is feasible to convert between mass- and volume-based distributions via particle density. However, differences in particle density, from product to product, or worse across a measured particle size distribution make such calculations product-specific and potentially problematic.
And, of course, no conversion from GPSD to APSD will address the issue of drug specificity.
The bottom line
Laser diffraction and cascade impaction are highly complementary for nasal drug product development, not interchangeable. Each has an important and distinct role to play.
Laser diffraction is a useful tool for assessing the impact of formulation and device parameters on likely deposition in the nasal cavity.
Cascade impaction is the most relevant method for quantifying the risk of off-target drug delivery.
The dataset we began with illustrates just how different the aerodynamic behaviour of particles can be from what we might infer from GPSD test results, and the corresponding impact when it comes to quantifying the proportion of the dose that lies in the sub-10 microns size range. This is a major limitation of using laser diffraction to assess pulmonary deposition and explains why we rely on cascade impaction instead, both for nasal and orally inhaled drug products. If you’re interested in reading more about nasal drug product testing, then try our article, Improving the Utility of In Vitro Methods for Intranasal Drug Delivery. Alternatively, look out for future blogs where we’ll be discussing issues such as automation to improve the productivity and repeatability of testing.
*Thanks to ‘Potts et al’ for permission to use this data which forms part of an interesting paper delivered at RDD 2023. Potts, J.C. et al. ‘Investigations into the Relationship Between Spray Dried Powder particle Size and Deposition in Nose and Lung Analogues when Actuated from a Nasal Device.’








