Exploring the intricacies of NGI design, the critical factors that control aerodynamic flow regime, the importance of calibration, and the implications of deviating from rigorously established, consistently maintained specifications.
It’s the end of the year 2000 and a new multistage cascade impactor is debuting, the first for decades.
It’s the NGI, an impactor designed by those at the forefront of inhaler development, to meet their evolving needs for aerodynamic particle size distribution (APSD) measurement. It will go on to dominate the orally inhaled and nasal drug product (OINDP) testing landscape.
We touched on the merits of the NGI relative to other impactors in an earlier blog but today we’re taking a deeper dive into the features that define its performance. Our aim is to provide insights on this critical item of equipment that is central to OINDP testing, because understanding the design intent for the NGI, and how it was met, is key to determining whether your NGI will be fit-for-purpose.
Developing the NGI
The consortium responsible for the NGI included representatives from 17 leading pharmaceutical companies. These companies represented the bulk of the OINDP industry, at the time, and most were predominantly using the Andersen Cascade Impactor (ACI) for testing. The ACI has its merits, but the experts had certain requirements for their new cascade impactor:
- A sub-30 minutes cycle time with a configuration suitable for automation
- Calibrated operation over 30 – 100 L/min, for metered dose and dry powder inhaler (MDI and DPI) testing (the extension to 15L/min for nebuliser testing came post-launch, as explained below)
- Steep stage efficiency curves with minimal stage overlap
- A minimum of five stages with cut-off diameters in the 0.5 to 5 µm range
- Inter-stage or wall losses of <5% on any individual stage and 5% overall to ensure a robust mass balance.
Ease-of-use, design and manufacturing standards were also a concern, but the specificity of this list is noteworthy. It came from an expert team, well-versed in OINDP testing working to improve data quality and impactor usability.
After intensively testing many prototype concepts a final design was agreed upon and the NGI was launched in Dec 2000.
Meeting the design brief
A striking difference between the NGI, relative to the ACI, is its horizontal planar layout, a critical feature with respect to ease-of-use. The nozzle assemblies are held in a single seal body, while the tear-shaped impaction cups slot into a single tray. A simple handle mechanism clamps the impactor together for leak-free operation. Relative to other impactors, it’s a design with fewer parts that is quicker and easier to use.
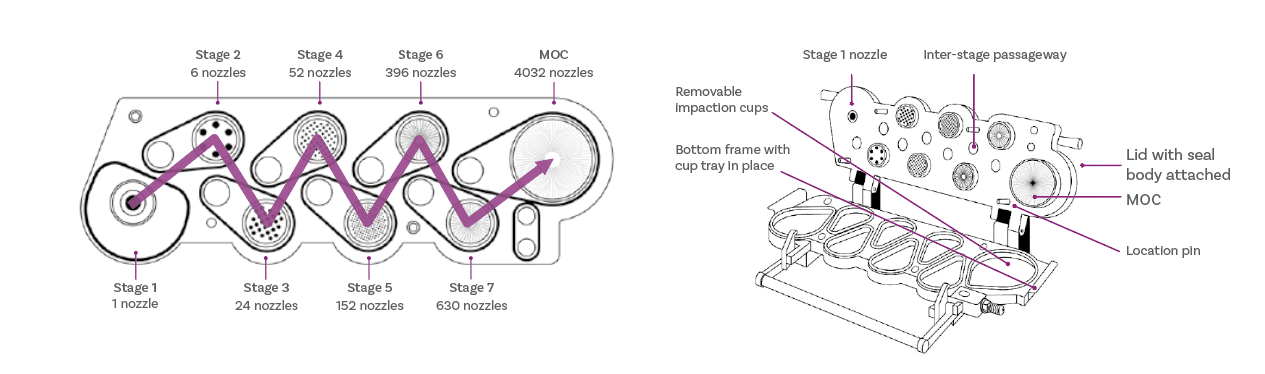
At the same time, it’s a design with rigorously optimised aerodynamic performance. Every material, surface finish, internal geometry and seal was specified with that in mind, both to shape that performance and maintain it. Any deviation from the specified design consequently carries hard to quantify risk, whether a change is intended or inadvertent due to a lack of appreciation of the original design. Even minor adjustments to the NGI may lead to problems such as higher interstage losses, altered thermal mass and/or failure to adhere to the archival calibration data that was established.
Focusing on aerodynamic flow
Critical dimensions that define the aerodynamic performance of the NGI include nozzle number, nozzle spacing and the nozzle-to-plate (or jet-to-plate) distance, which are all correlated to the reciprocal design of the collection cups. The chosen design optimises these with respect to flow regime, the prevention of excessive crossflow, and desirable impaction behaviour. It also has the rigidity, sealing mechanisms, and precision manufacturing tolerances needed to maintain performance over many years, as perfectly illustrated by Copley’s very first NGI, serial number 1, which is still in active use today.
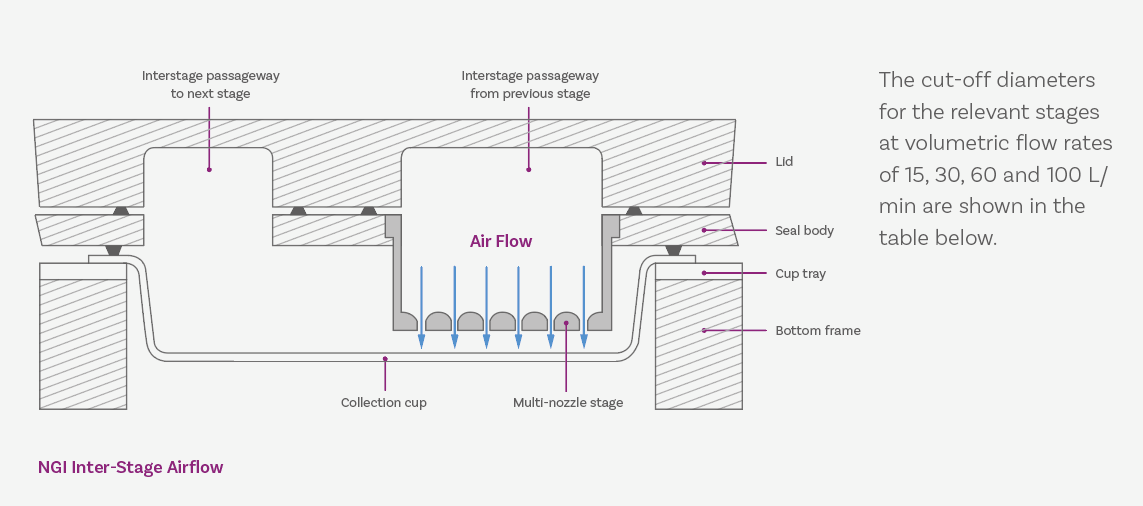
The figure above shows the pathway of air through each individual stage of the NGI. This pathway minimises particle carryover and interstage losses to the extent that only the impactor cups need changing between runs. By simply lifting the tray containing all the cups and replacing with a new set, an analyst can move onto the next measurement.
Calibrating Performance
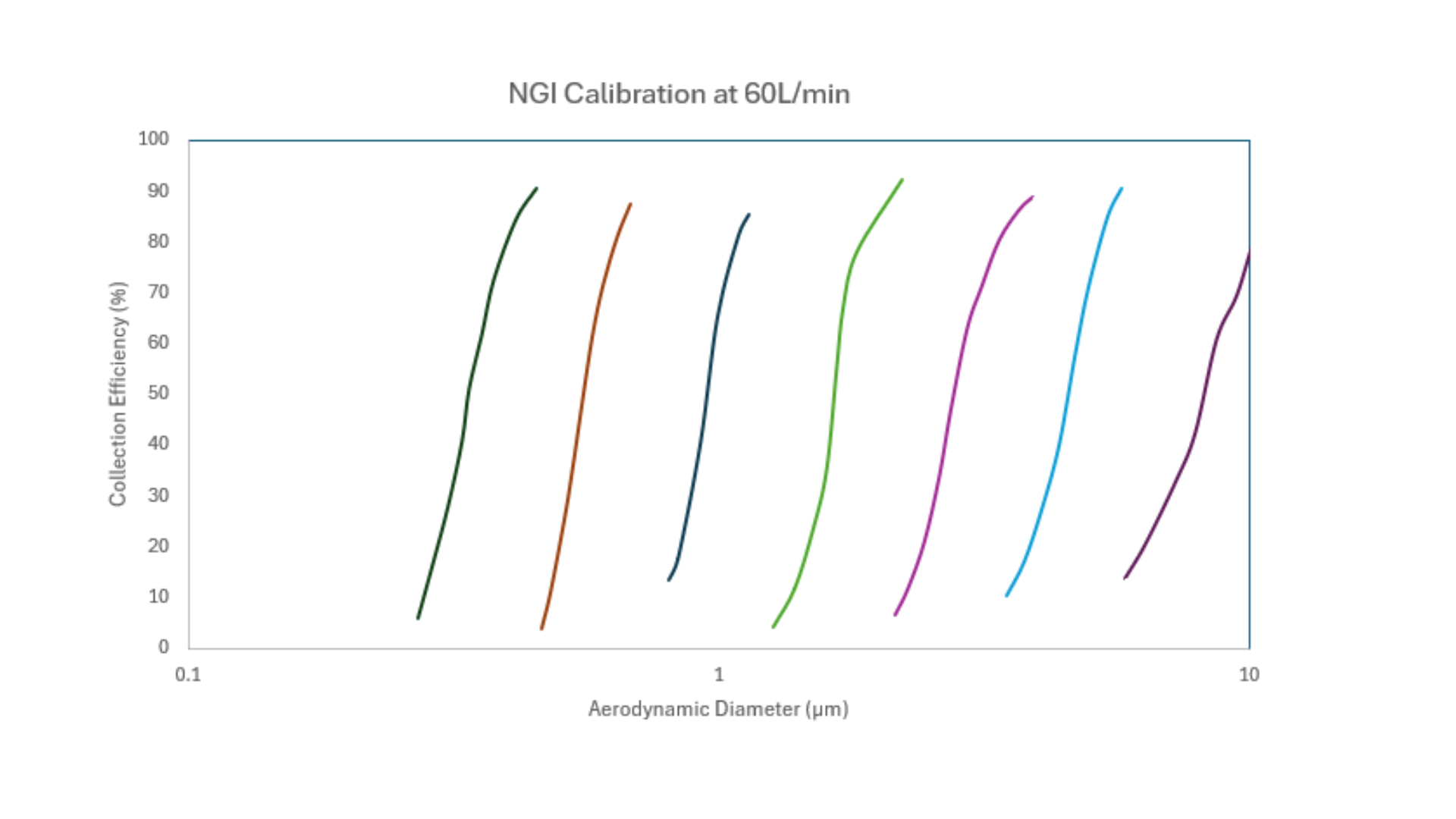
To confirm performance, every stage of an ‘archival’ NGI was calibrated at 30, 60 and 100 L/min. As we’ve discussed in an earlier blog, calibration is not a routine exercise for cascade impactors. This aim of this one-off exercise was to provide a secure foundational reference for ongoing use of the specific NGI design used for this archival calibration. The ‘archival’ NGI had nozzle dimensions close to the nominal value, i.e. towards the centre of the range associated with the manufacturing specification. Calibration was carried out in accordance with Good Laboratory Practice.
Stage | 15 | 30 | 60 | 100 | L/min |
1 | 14.10 | 11.72 | 8.06 | 6.12 | microns |
2 | 8.61 | 6.40 | 4.46 | 3.42 | microns |
3 | 5.39 | 3.99 | 2.82 | 2.18 | microns |
4 | 3.30 | 2.30 | 1.66 | 1.31 | microns |
5 | 2.08 | 1.36 | 0.94 | 0.72 | microns |
6 | 1.36 | 0.83 | 0.55 | 0.40 | microns |
7 | 0.98 | 0.54 | 0.34 | 0.24 | microns |
The results demonstrate the extent to which this NGI meets its brief. Stage efficiency curves have been proven to be steep with minimal overlap (left); and there are five stages with cut-off diameters demonstrated to be in the sub-5 µm range across the 30 – 100 L/min flow rate range.
The pharmacopeial requirement for operation at 15 L/min came after the NGI launch, with the refinement of nebulizer testing. In response a further calibration of the archival NGI was carried out (see data above).
NGIs supplied by Copley are manufactured to the exact same specification as the archival instrument meaning that users have the benefit of calibrated performance from 15 L/min to 100 L/min. By interpolation analysts can securely determine stage cut-off-diameters at flow rates anywhere within this range, and we offer routine stage mensuration to make sure they stay that way. This is essential to validate the critical dimensions of an NGI relative to the design specification and safeguard consistent performance.
The pharmacopoeias uniquely specify the NGI for the APSD measurement of nebulisers, with many in the industry also opting to use it for Soft Mist Inhalers (SMI) for analogous reasons. It is the only cascade impactor specified for testing all types of OINDP. Furthermore, the NGI’s amenability to automation has led to a range of tools for drug recovery and productivity is typically relatively high. As a result, where there is the freedom to choose, analysts across the globe typically opt for the NGI in preference to other impactors.
The bottom line – achieving the calibrated performance of the NGI relies on precision manufacture to an exacting specification and maintaining performance through regular stage mensuration. An NGI with subtle deviations from the original design may cause immediately obvious performance differences or worse, create issues that only emerge over time as the impactor responds poorly to ongoing use.
Follow up here to find out more about the NGI’s performance and use.








