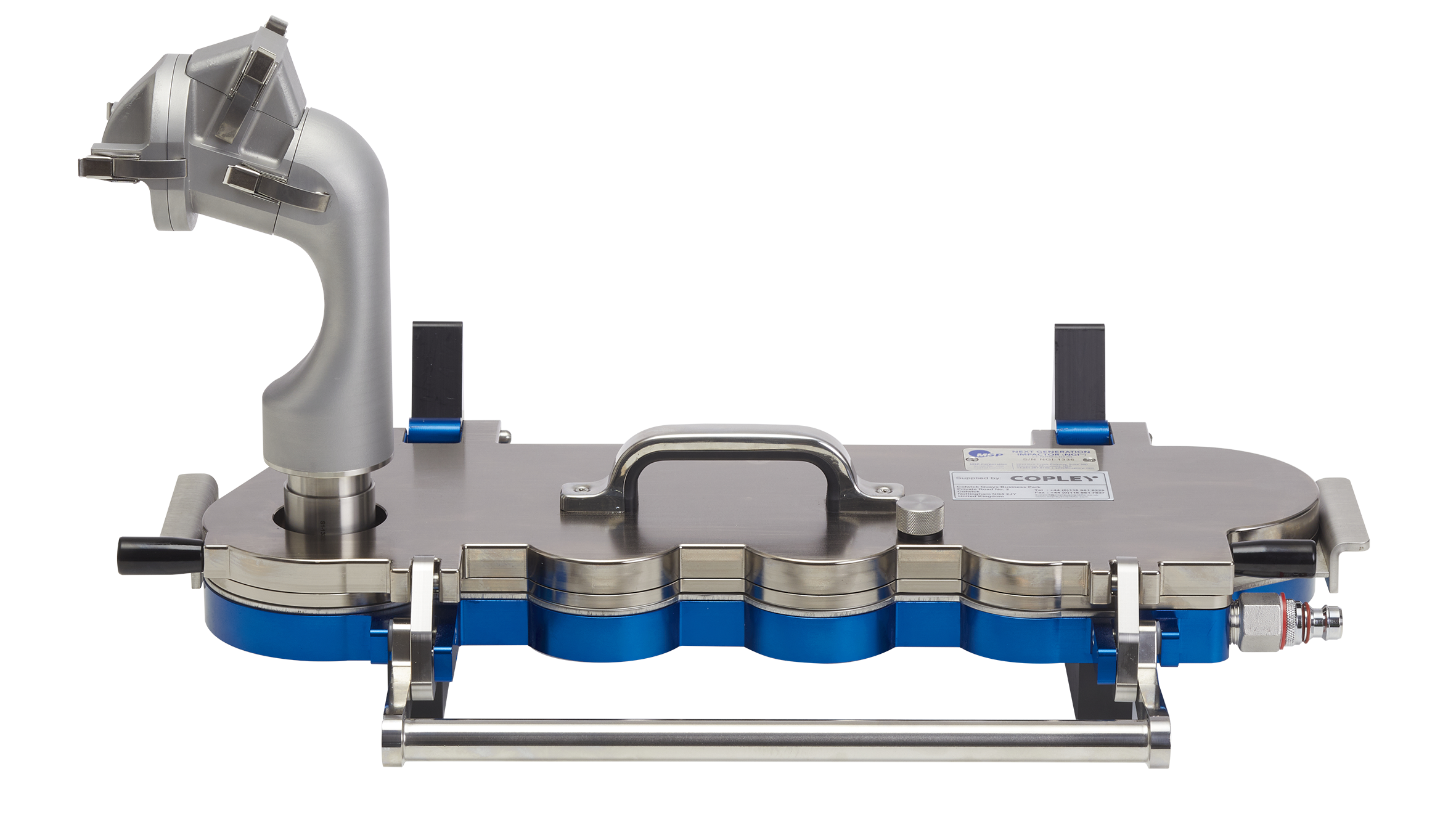
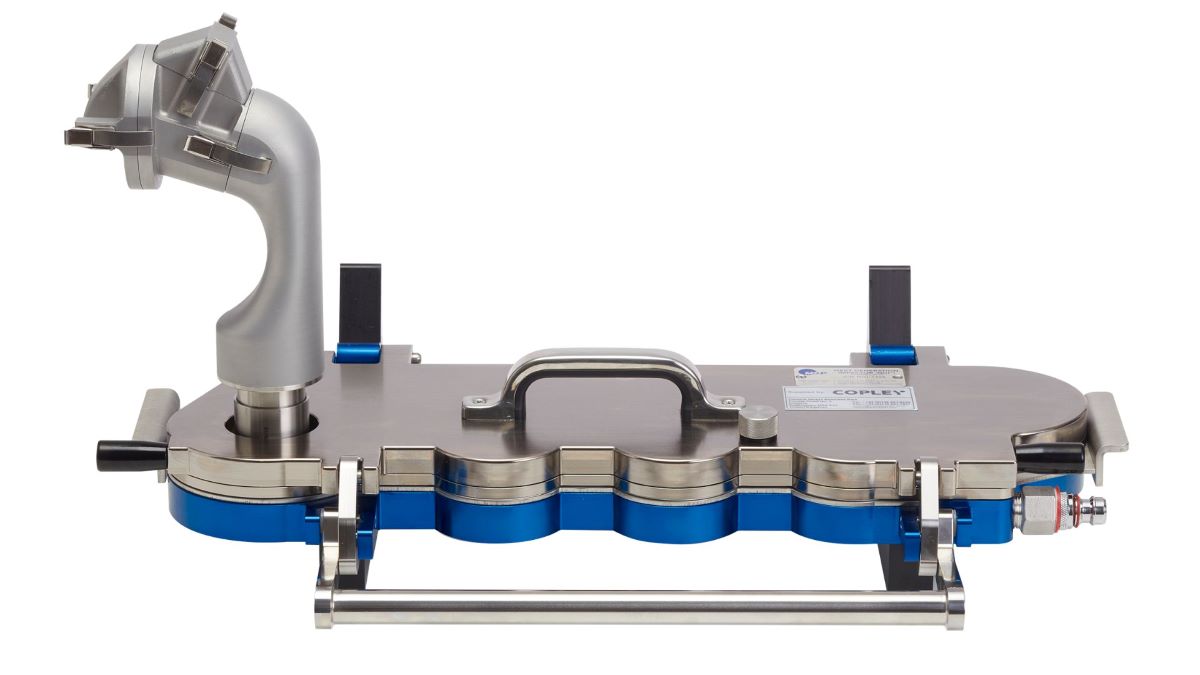
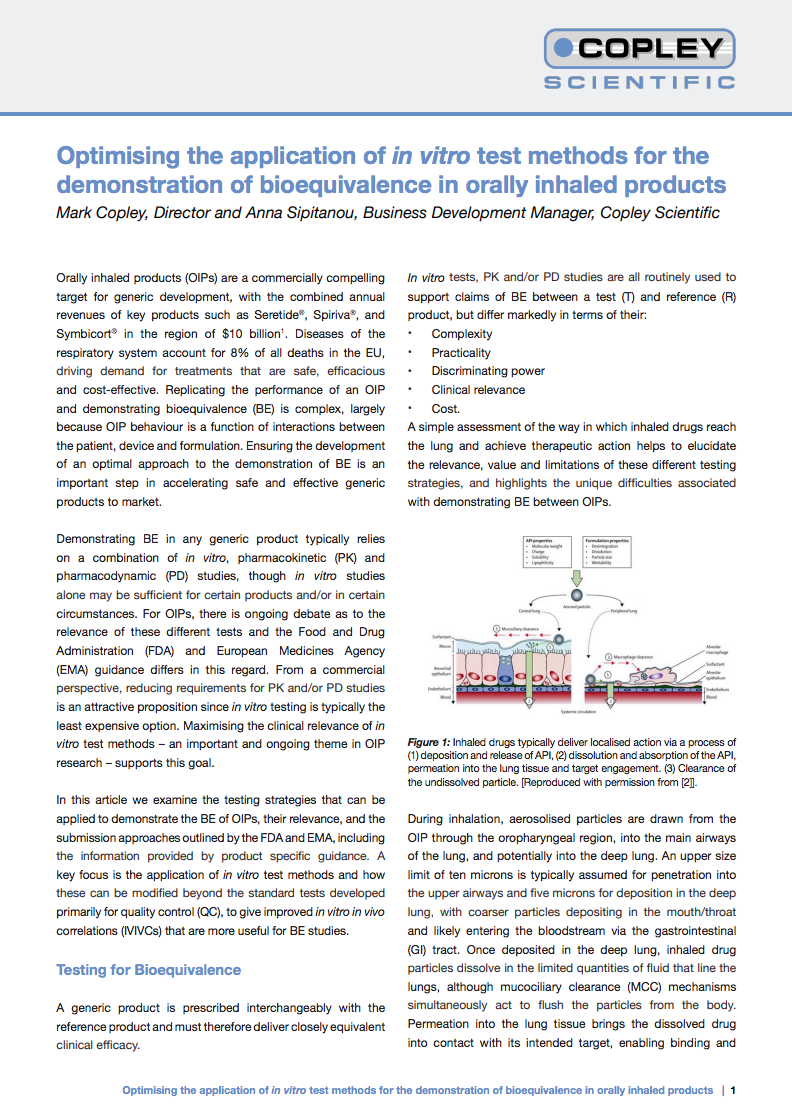
Regions represented by the Alberta Idealised Nasal Inlet AINI
The AINI accurately mimics deposition behaviour in each region, allowing the collection of drug samples that reflect the corresponding fraction of the dose for analysis.
The AINI has a four-region resolution:
- the vestibule
- the turbinates
- the olfactory region
- the nasopharynx
The AINI is easily separated into its component parts to enable drug recovery and assay for each individual area. The AINI comes complete with mensuration and test certificates.
The nasal model we offer was developed from extensive research into typical patient populations including information provided by CT and MRI scans, direct observation of living subjects and data in the archival literature. More information and references are available on request.
System for Improved In Vitro-In Vivo Correlations (IVIVCs) of Inhaled Drug Products
Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out More