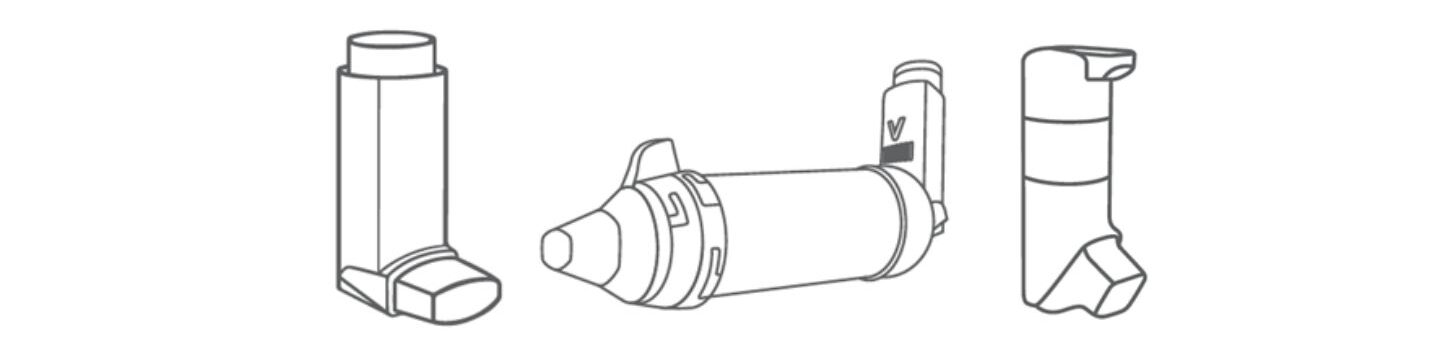
There’s no shortage of device types when it comes to inhaled drug delivery. Here’s a round-up with an overview of advantages and disadvantages of different inhalers.
In an earlier blog we looked at the intrinsic challenges of inhaled drug delivery, focusing on the need to consistently deliver extremely fine particles (sub-5 microns) in the face of variability in patient physiology and technique. The diverse array of commercially available inhaler device types is a direct response to that challenge. Inhaler devices such as nebulisers, metered dose inhalers (MDIs), dry powder inhalers (DPIs) and newer innovations such as aqueous droplet inhalers (ADIs – or soft mist inhalers) extend efficient inhaled drug delivery to the broadest range of drug substances, and patient populations.
Here we look at some of the factors that encourage the choice of one inhaler device relative to another. More than 400 million MDIs are sold annually (based on latest available data)i. The value of the DPI market is higher, though the number of doses delivered is perhaps around a quarter of the MDI counti. And despite being the oldest inhaled drug delivery technology, nebulisers are having somewhat of a renaissanceii. What makes different inhaler devices more or less suitable for different applications, why are some used more than others and what factors are shaping today’s choices?
What are the key advantages of MDIs?
MDIs are small, inexpensive – particularly when assessed on a per unit dose basis – convenient for the user and suitable for the delivery of many types of drugs. This is an impressive slate of benefits that more than justifies their frontline position in inhaled drug delivery. Cost is especially important given the growing prevalence of respiratory disease. In developing countries, MDIs preserve quality of life for millions of people suffering from illnesses such as asthma and chronic obstructive pulmonary disease (COPD), at an accessible cost.
What are the key disadvantages of MDIs and how do we address them?
The two key disadvantages of MDIs are the need for coordination during use and environmental impact.
Correct use of an MDI requires a patient to inhale deeply as they press down on the cannister to actuate the device. With practice many patients can successfully apply this technique but for some – for instance, paediatrics and geriatrics – the strength and coordination required may prove a bar to effective use.
Add-on devices – spacers and valved holding chambers (VHCs) – are an established solution. Actuation into an add-on device creates an aerosolised cloud that the patient then inhales. The need for strict coordination is eliminated along with the so-called ‘Cold Freon®’ effect which is when plume impact and propellant evaporation combine to irritate the back of the throat and trigger coughing, compromising drug delivery. Using add-on devices, MDIs can therefore be used to successfully treat a far wider range of patients. Breath-actuated inhalers are an alternative that, as the name suggests, release the dose automatically as the patient inhales, thereby similarly eliminating the need for coordination.
Environmental impact is a tougher nut to crack. The amount of propellant associated with each MDI dose is very small, but the cumulative impact is significant: GSK have estimated that use of their MDIs alone is responsible for 5.0 million tonnes CO2e (carbon dioxide equivalent) per annumiii. The solution here is reformulation with propellants with a lower global warming potential (GWP). Most major MDI manufacturers already have aggressive timelines in place to switch, with HFA 152a and HFO 1234ze the leading low GWP candidates. Reformulation will require the careful consideration of, for example, formulation stability and properties, materials of construction and manufacturing processes but new products are expected as early as 2025.
What are the unique benefits of DPIs?
With most DPIs, dose delivery is driven by the patient inhaling through the device. This eliminates any requirement for effective patient coordination, or indeed propellant. Here in the UK, the NHS is working to switch patients from MDIs to DPIs for precisely this reason; to reduce the environmental impact of treatmentiv. The sterility of dry formulations is also advantageous when it comes to multidose products. Storage and shipping are both substantially easier for dry formulations which can be especially useful for drug delivery in remote locations and/or developing countries.
What are the key drawbacks of DPIs and how do we address them?
In the absence of ‘active’ drug delivery, certain patients are simply unable to apply sufficient energy to successfully empty a DPI device and disperse the dose. Physiology and disease state are influential but, crucially, this drawback is a major limitation to using DPIs for the delivery of reliever medications.
Device design can help. The pressure drop across a DPI device is critical when it comes to patient use with higher pressure drops necessitating higher inspiratory force. Designing devices that achieve dose dispersion under conditions readily applied by specific patient groups is an ongoing goal, underpinned by testing under representative inhalation profiles.
Design can also help when it comes to reducing susceptibility to humidity, a second drawback of DPIs. Protection from the environment, for example, via packaging design is helpful but problems can also arise from poor technique. The common practice of accidentally exhaling into a DPI prior to use introduces moist air that can cause the formulation to clump, compromising dose delivery. Longer mouthpieces and other adaptations can lead to more robust performance, but again representative testing is essential to scope the problem and assess mitigation strategies.
Finally, it is worth noting that DPIs present multiple manufacturing challenges. The devices themselves tend to be relatively complex but powder handling is the bigger issue. It is difficult to maintain high intra- and inter-batch uniformity when preparing and dosing/filling small volumes of fine powders, often with necessarily well-controlled morphology.
What specific applications are nebulisers used for?
Relative to inhalers, nebuliser are big and bulky. They also require a source of power or compressed air. As a result, use has historically been confined to clinical settings where nebulisers are relied on for inhaled drug delivery to critically ill patients in intensive care units (ICUs) and/or those requiring mechanical ventilation.
However, in recent years devices have become smaller and more portable. Today we have jet, ultrasonic, and vibrating mesh designs, each suited to different drug formulations. Fundamentally, since many drugs are routinely formulated as aqueous solutions, nebulisers have broad application, particularly when considering biologics. Going forward, antibiotics, vaccines and even stem cells may therefore become common features of the nebuliser formulation market along with new therapeutics for the treatment of key illnesses of the ICU such as acute respiratory distressii.
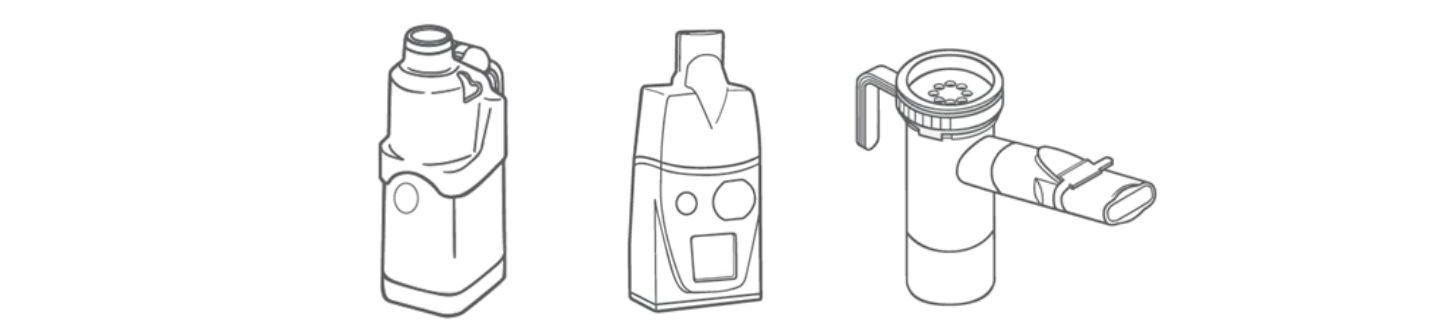
What about new inhaler device types?
While most new devices fall into one of the traditional classifications, ADIs are different. They actively aerosolise a liquid formulation, typically using technology akin to that deployed in nebulisers, to deliver a soft mist that is inhaled by the patient, as with an MDI.
Key advantages include no propellant, ease of use, and, critically, much higher deposition within the lung than alternatives. These are significant attractions, and since this technology uses aqueous formulations, there is potential for application to a wide range of drugs. In the future ADIs may become increasingly common, although cost remains a significant barrier to overcome.
This brief overview highlights some of the factors that shape device choice when it comes to OINDP development: formulation properties, therapeutic regime, patient characteristics, and of course cost. In the new year we are going to begin a series of blogs looking in more detail at how test methods are tailored to specific device types but in the meantime watch out for our round-up from DDL – coming soon!
iA.R. Clark, J.G. Weers, and R. Dhand ‘The Confusing World of Dry Powder Inhalers: It Is All About Inspiratory Pressures, Not Inspiratory Flow Rates’ J Aer Med and Pulm Drug Del. Vol 33, No 1. Available to view at: https://www.liebertpub.com/doi/10.1089/jamp.2019.1556
iiS.D. McCarthy, H.E. Gonzalez and B.D. Higgins ‘Future Trends in Nebulized Therapies for Pulmonary Disease’ J Pers Med 2020 Jun; 10(2): 37
iiiGSK ‘Our pathway to net zero impact on climate’ Available to view at: https://www.gsk.com/media/9967/pathway-to-net-zero-impact-on-climate-2022.pdf
ivNHS ‘Delivering a Net Zero Health Service’ Available to view at: https://www.england.nhs.uk/greenernhs/wp-content/uploads/sites/51/2020/10/delivering-a-net-zero-national-health-service.pdf








