Answering the basic and not-so-basic questions associated with this core technique for testing orally inhaled and nasal drug products.
Ever wondered why cascade impaction is critical for orally inhaled and nasal drug products (OINDPs)? How it works? Why it’s not fully automated? Then read on. We’ve been working with this pivotal technique for decades and there are certain questions that come up time and again. Here’s our top 10:
1) Why is cascade impaction used specifically for inhaled drugs?
Three distinct attributes set cascade impaction apart from all other particle impaction sizing techniques in terms of relevance for orally inhaled products (OIPs):
(1) The ability to generate particle size data for the active pharmaceutical ingredient (API), rather than the whole formulation
(2) Measurement of aerodynamic particle size distribution (APSD), an intuitively relevant metric for inhalation
(3) Good resolution in the 0.5 to 5 µm range of interest for drug delivery to the lung
For nasal drug products cascade impaction is similarly well-matched to requirements to assess the risk of deposition in the lung, rather than the nasal cavity.
2) Does a cascade impactor work like a sieve (because it looks a bit like one)?
No, cascade impactors size fractionate a dose on the basis of particle inertia rather than by sieving.
Through the impactor, nozzle size and total nozzle area decrease with stage number. This means that air velocity progressively increases, since volumetric air flow rate is constant. At each stage, particles with sufficient inertia break free of the air stream and impact on a collection surface; the remainder flow through to the next stage. In the early stages of the impactor, when air velocities are lower, the relatively large particles are collected, since inertia is a function of particle impaction mass and velocity. However, as nozzle area decreases, and velocity correspondingly increases, smaller and smaller particles acquire sufficient inertia to break free of the airstream and impact on to the collection surface. Each subsequent stage therefore has a smaller stage cut-off value; each collects a smaller sized fraction. The recovery and assay of each fraction produces APSD data for the API.
Watch our animation to see cascade impaction in progress:
3) Does a cascade impactor simulate deposition in the lung?
Not directly. Lung deposition is highly complex and influenced by unique patient physiologies, disease state and prevailing in vivo conditions. However, by measuring particles moving in air, under somewhat relevant flow conditions, impacting on a surface, cascade impaction applies comparable conditions, thereby fulfilling requirements for a standardised, reproducible in vitro technique for investigating deposition behaviour.
4) Why are there different cascade impactor designs in use?
That’s largely historical. The Next Generation Impactor (NGI), which has been commercially available for just over 30 years now, is the result of a consortium effort to develop a cascade impactor tailored to the needs of the inhalation community; it tends to be the impactor of choice for new products. However, the Andersen Cascade Impactor (ACI), which originated in the air sampling industry, is still widely used; alternatives such as the Glass Twin Impinger (GTI) and Multi-Stage Liquid Impinger (MSLI), less so. When testing generics, using the same cascade impactor that was used in the original product development is preferred which creates a lag in terms of the uptake of newer designs.
5) Why is test flow rate so important?
The volumetric flow rate of air through a cascade impactor determines the speed at which the air, and by extension particles, flows through the nozzle of any individual stage. Stage cut-off values are therefore a function of volumetric air flow, rather than fixed. This is why test flow rate is important. It needs to be both known and constant.
6) What other issues do I need consider in method development?
Many, but there is plenty of guidance to help. We often use this fishbone diagrami to highlight potential sources of variability in cascade impaction measurements, all of which should be considered as part of method development. You can see these are grouped as related to Man (the operator), Measurement (the technique, including assay), Material (the product) and Machine (the cascade impactor).
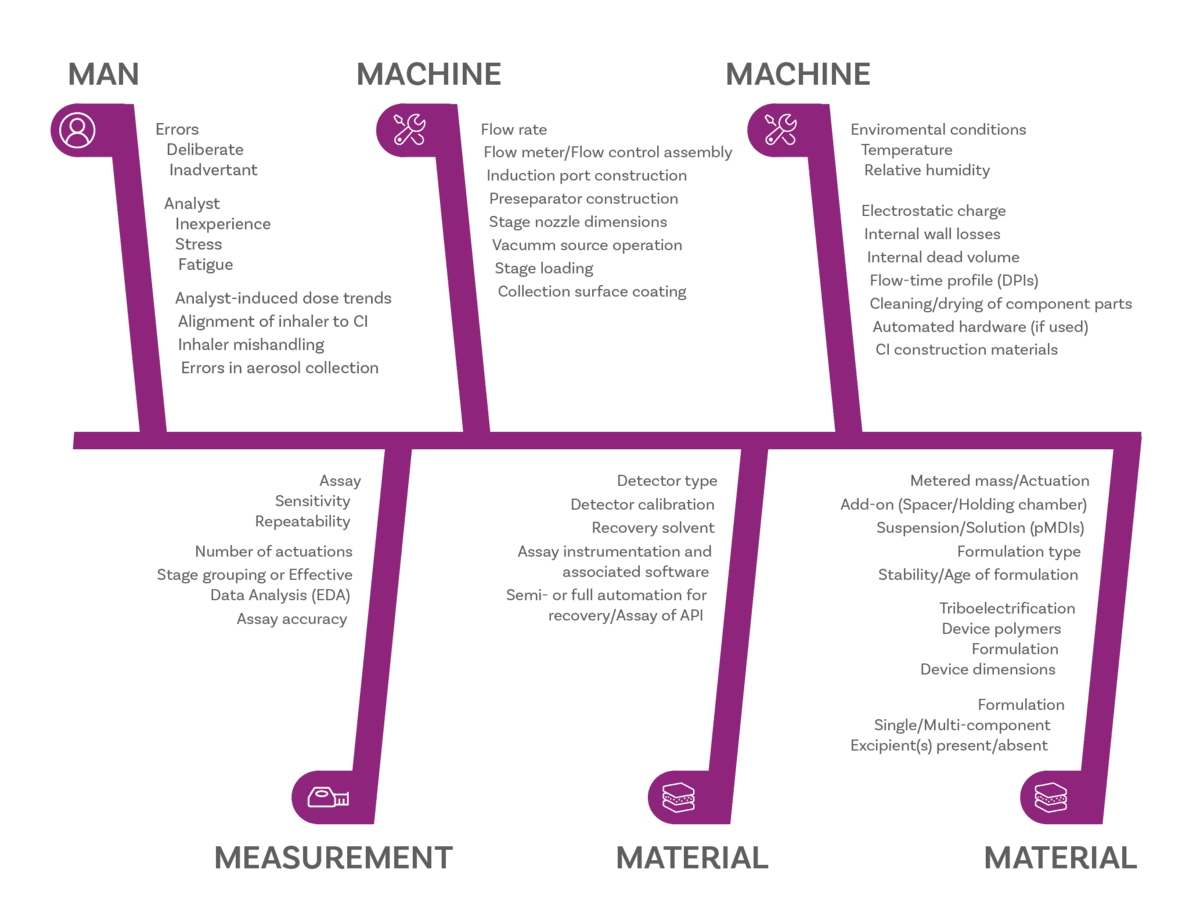
Sources of potential error in APSD measurement ii
7) How do I know my cascade impactor is working properly?
The performance of a cascade impactor is defined by its critical dimensions, including the diameter of every nozzle, so these are routinely re-measured to check that they lie within acceptable tolerances. This is ‘stage mensuration’ and the frequency with which this is required will depend on usage. More routinely, pressure testing and the measurement of pressure drop across the impactor are good practice and will help to detect a problem.
8) What other equipment do I need to develop a cascade impactor set-up?
For the simplest compendial test set-up, as used for metered dose inhalers (MDIs) you’ll need a vacuum pump to draw air through the impactor, a flow meter to determine volumetric air flow rate, an induction port to introduce the dose into the impactor, and a mouthpiece adapter to interface product and induction port (see below); a flow controller is additionally required for certain types of MDI/compendial methods. For dry powder inhalers you’ll also need a critical flow controller.
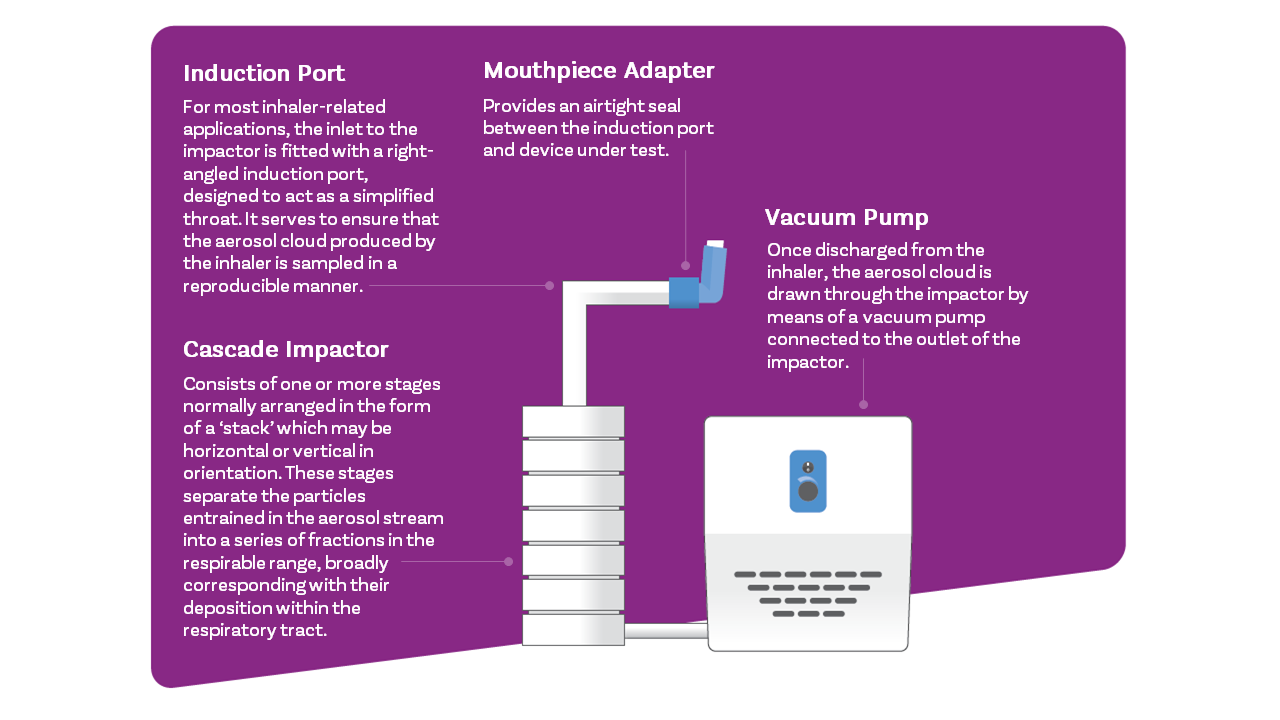
For improved in vitro-in vivo relationships compendial test set-ups may be refined using items such as breathing simulators, mixing inlets and anatomically realistic induction ports, (e.g. the Albert Idealised Throat).
9) Are automated versions available?
End-to-end cascade impaction is not amenable to automation and looking back at the fishbone diagram it’s easy to see why. There are too many variables associated with method development to make complete automation economically attractive. Almost every OINDP will have a cascade impactor test set-up unique to it, in some way.
That said, the process is highly amenable to semi-automation. Most practitioners automate at least a few repetitive tasks, notably device actuation and processes associated with drug recovery, and there are off-the-shelf solutions for that. These can deliver a great return on investment by improving productivity, freeing up analysts for higher value work and reducing the risk of out-of-specification results; they can also alleviate health and safety concerns, a major gain.
10) How do I process my results to generate the metrics I need?
Invest in dedicated, validated software. The best enable easy input of the large amounts of assay data that cascade impaction produces and rapidly converts it into the metrics you need, using prescribed pharmacopeial methods. Look out for flexibility, ease-of-use and regulatory compliance.
If this has whetted your appetite for more information on cascade impaction, then sign up to hear about future blogs in which we’ll be delving into all these topics in further detail.
i Bonam, M. Christopher, D. Cipolla, D. Donovan, B. Goodwin, D. Holmes, S. Lyapustina, S. Mitchell, J. Nichols, S. Pettersson, G. Quale, C. Rao, N. Singh, D. Tougas, T. Van Oort, M. Walther, B. and Wyka, B. ‘Minimizing Variability of Cascade Impaction Measurements in Inhalers and Nebulizers’ AAPS Pharm Sci Tech AAPS, 2008.
iiJ.P. Mitchell. The good cascade impactor practice (GCIP) concept. A systematic approach to risk management of erroneous measurements in the assessment of inhaler emitted aerosol aerodynamic particle size distributions (APSDs), Inhalation, 8(2), April 2014.








