An introduction to the challenges of drug delivery to the lung with a complementary look at intranasal drug delivery.
We breath in and out up to 20,000 times a day introducing oxygen into our bodies via airways and alveoli with a combined surface area in excess of 100mi. Inhaled or pulmonary drug delivery takes drugs straight to where they are needed for respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD), but this large surface area is increasingly a target for systemic drug delivery too. Here we look at how drugs are delivered to the lung and at the role of the inhaler, the formulation, and the patient in achieving success. We conclude with a complementary look at drug delivery via the nasal cavity.
Particle size: the key to entry
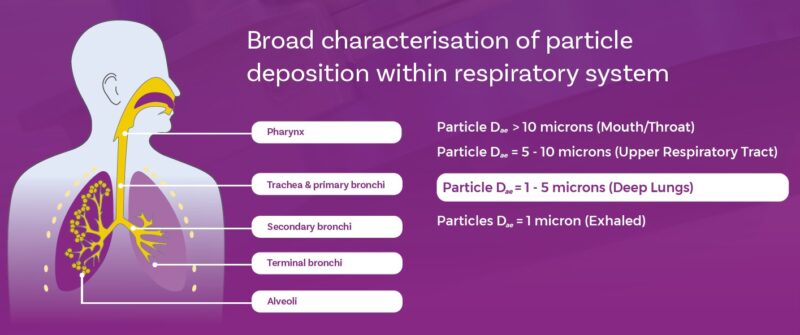
The deposition behaviour of particles drawn into the body during oral inhalation is complex but defined by particle mass and flow velocity, which together determine particle inertia. By controlling particle size – via formulation and device design – we influence deposition and, by extension, the success of drug delivery.
Larger particles, those with an aerodynamic particle size in the order of 5 to 10 microns, will tend to deposit in the upper respiratory tract via impaction or interception. Finer particles, with less inertia, are carried further into the lower respiratory tract, with 5 microns typically taken as an upper limit for dose delivery to the lung. Successive branching within the lung results in particles navigating ever narrower passageways and only those with minimal inertia will reach the deep lung. The delivery of particles with an aerodynamic particle size distribution centred around 1-2 micron is routinely a target for deposition in this region.
You’ll notice aerodynamic highlighted in the paragraph above. This is because there are many ways to evaluate particle size, including techniques such as laser diffraction and imaging, but aerodynamic particle size is uniquely relevant for inhaled drug delivery. The associated science and understanding are therefore built on aerodynamic particle size data measured specifically via the technique of cascade impaction. Look out for future blogs discussing this important issue.
Let’s take a closer look at how particles in the sub-5 micron range behave to get a clearer idea of the implications of this requirement to deliver such fine particles.
As particles become smaller and, by extension, lighter (assuming constant density), the gravitational forces acting on them decrease relative to the magnitude of cohesive forces, such as van der Waals, that draw them together. Furthermore, specific surface area rises significantly. This means that the discrete particles we are trying to deliver will be prone to aggregation and, if hygroscopic, will easily absorb water, potentially making them larger and/or ‘sticky’.
This simple overview offers insight into the basic challenge of inhaled drug delivery and explains why controlling particle size is a crucial first step.
Device and formulation: matched for success
Dispersion at the point-of-use maximises the respirable dose delivered to the patient and is a feature of all orally inhaled products (OIPs). Devices including nebulisers and inhalers break up the formulation, which can be a liquid or powder, via energetic processes that involve applying shear and inducing collision. There’s an array of devices on the market because there’s an array of formulations, with each exhibiting a unique response to the conditions applied by a specific device.
Let’s illustrate this specificity by examining the mechanism of operation of a metered dose inhaler (MDI). MDI formulations are liquid – suspension or solution – and incorporate a propellant. Upon actuation the propellant vaporises rapidly, separating it from the rest of the liquid formulation as it does so, dispersing it to a target particle size distribution. From this viewpoint it’s easy to see that the physicochemical properties of the formulation – viscosity, interfacial tension, polarity – will influence how it responds to this propellant-driven process. And that the same formulation will perform differently in a different device if, for example, it changes the release rate of the propellant or forces the formulation through different geometry.
The point here is that we have two sets of variables to manipulate to reach our goal of delivering particle of respirable size. One set relates to the formulation, the other to the device. We can change excipients and composition and/or incorporate additives. We can change materials of construction, internal geometry, and patient interface. But the two sets are not completely independent: robust product optimisation calls for the careful consideration of interactions between the two.
The patient: an unknown variable
And then there’s the patient.
It’s evident from the preceding discussion that dose delivery with an OIP is going to be somewhat patient-dependent. The breathing profile applied – and by extension air flow and particle inertia – will vary. As will physiology, across patient populations and from individual to individual.
To use an OIP patients may be asked to breath normally (nebuliser), slowly and steadily (MDI), or quickly and deeply (dry powder inhalers (DPIs)). Such instructions mean different things to different patients, and in any event may be significantly impacted by impaired lung function. And there are other aspects of technique that can be a problem. Estimates suggest that between 14 and 22% of patients routinely exhale into their DPI prior to use thereby introducing moist air that could promote formulation aggregationii and coordinating actuation and inhalation is a well-recognised issue affecting MDI use.
This is variability that we can’t completely eliminate, though we can minimise it through, for example, better patient training, and increasingly digital/connected devices. Fundamentally though we can reduce patient-to-patient variability by designing devices that are more robust or that are uniquely specified to meet the needs for specific patient populations. We can investigate this source of variability, be aware of it, and design for it to ensure more reliable therapeutic outcomes.
Intranasal drug delivery: somewhat similar, somewhat different
The role of the nose is very different to that of the lungs. It’s designed to warm, humidify, and filter incoming air. From a drug delivery perspective this can aid deposition. Generally, particles across the size range 10-150 microns will deposit in the nasal cavity with 40-100 microns a common mean particle size target for nasal spraysiii,iv. One of the issues with intranasal drug delivery is the avoidance of fine particles, a sub-10 micron fraction, since this will not be reliably filtered but will rather proceed into the lung entering the body via a different and/or undesirable route.
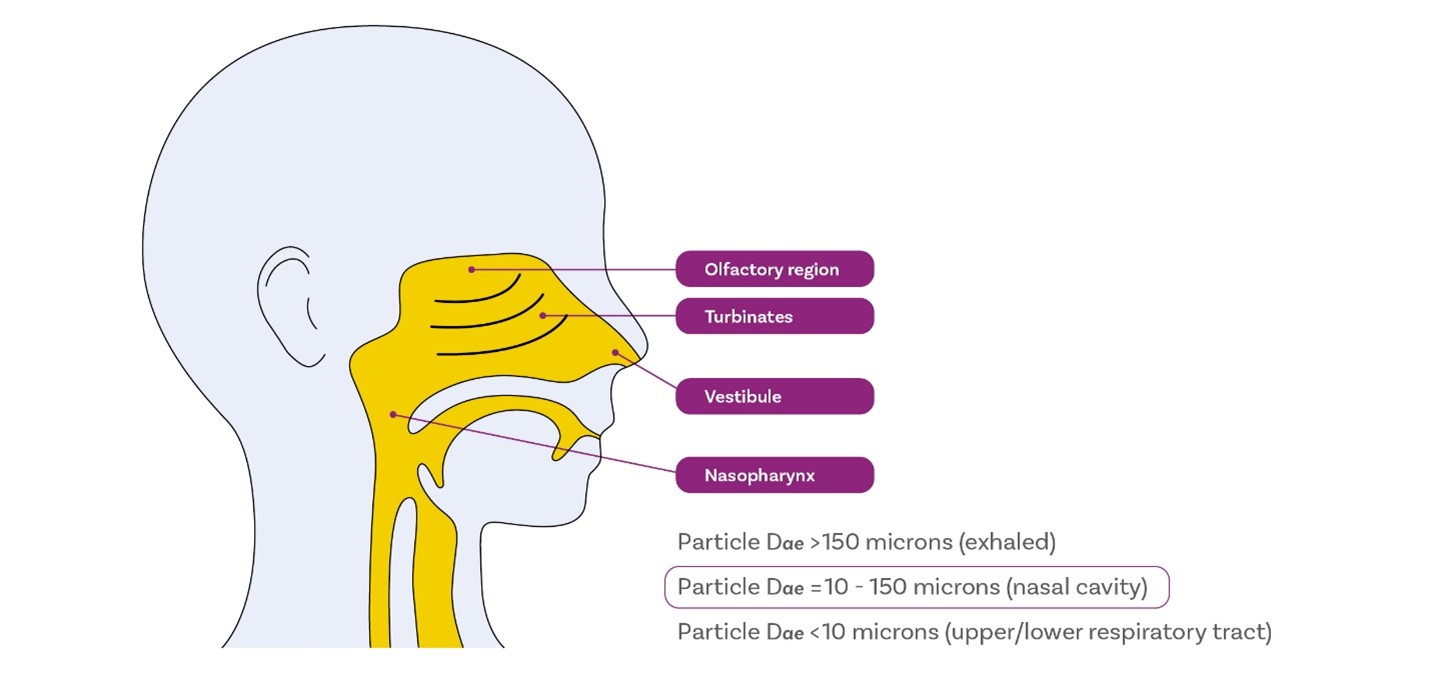
When it comes to intranasal drug delivery, we can therefore say it’s similar to pulmonary drug delivery due to the need to control particle size, and in terms of using a device and formulation together to do so, as well as the potential for patient variability – inhalation profile, insertion depth and angle. On the other hand, its different, and less demanding, in terms of the level of dispersion required. Untargeted deposition within the nasal cavity for topical action is relatively easy, compared with pulmonary drug delivery.
But, and it’s a big but, we’re increasingly looking to use the intranasal drug delivery for non-topical drugs and that’s a very different story. For this we need effective targeting and to overcome the nasal cavity’s highly effective mechanisms for dealing with contaminants. You can be sure we’ll be returning to this topic in future blogs.
Next up, however, its oral solid dosage forms. If you’d like further reading on inhaled drug delivery in the meantime, then why not try this article or look out for future blogs focussing on critical to quality pharmacopeial tests for OINDPs including insights into the central technique of cascade impaction.
i Breathe, the lung association. Breathing. Available to view at: https://www.lung.ca/lung-health/lung-info/breathing
ii M.S Holmes et al. An Acoustic-Based Method to Detect and Quantify the Effect of Exhalation into a Dry Powder Inhaler. JAMP 28(4) 2015, p247-253
iii M. Trenkel and R. Scherlieβ. Nasal Powder Formulations: In-Vitro Characterisation of the Impact of Powders on Nasal Residence Time and Sensory Effects. Pharamceutics 2021 Mar; 13(3): 385
iv D. Marx et al. Intranasal Drug Administration – An Attractive Delivery Route for Some Drugs. InTechOpen. Drug Discovery and Development. Published June2015. Available to view at: https://www.intechopen.com/chapters/48052