When it comes to nasal drug products, cascade impaction measurements are all about determining safety.
To date our discussions about cascade impaction have centred on its use for testing orally inhaled products (OIPs). For these products, cascade impaction data supports the targeting of drug delivery to the lungs. With nasal drug products, the goal is to avoid drug delivery to the lungs, but cascade impaction is still vital. Cascade impaction data directly underpins the safety of intranasal drug delivery by quantifying the risk of pulmonary drug delivery.
1. How cascade impaction supports nasal drug product development
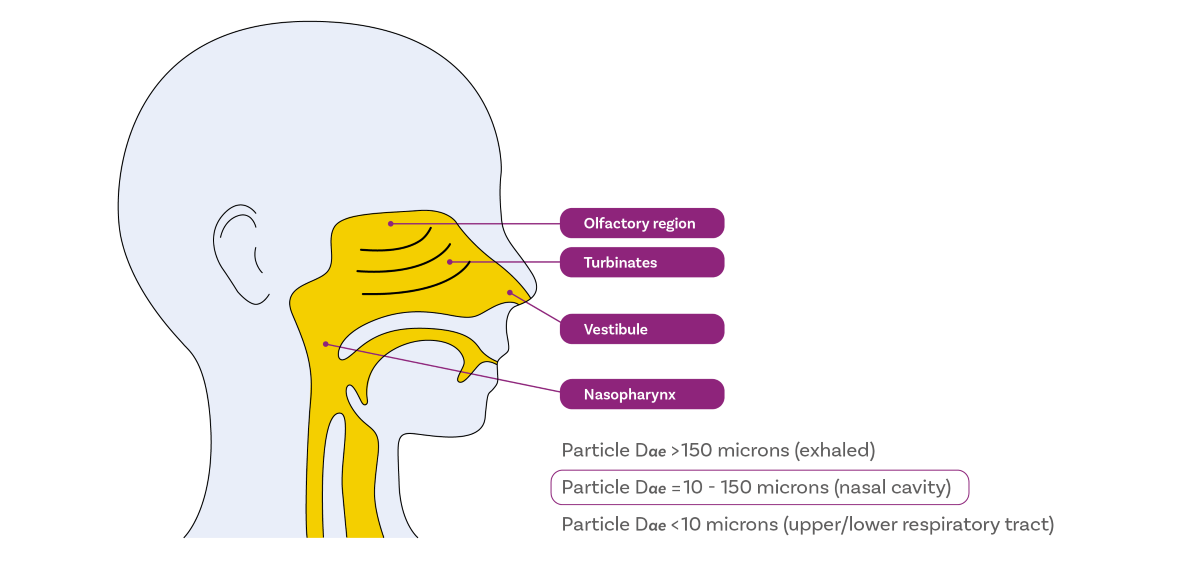
Our bodies are built for nasal breathing, that is for air to be drawn into the lungs via the nose. The nose warms and humidifies incoming air while simultaneously defending the rest of the respiratory system from contaminants and pathogens. Nasal mucociliary clearance is highly effective at capturing substances and particles and expelling them via sneezing, neutralising them using the nasal immune system or sweeping them towards the mouth, for swallowing or expectoration. Intranasal drug delivery safety relies on these mechanisms preventing the drug from being carried further into the body.
An earlier blog highlighted the centrality of particle size with respect to deposition behaviour in the lung and it is similarly influential for intranasal drug delivery. For nasal drug products, droplets or particles that are:
- Larger than ~150 µm tend to deposit at the front of the nose, with conditions unfavourable for drug uptake and a high risk of loss via dripping.
- Within 30 – 120 µm are optimal for deposition in the turbinates and olfactory region, the target regions for drug delivery due to their highly vascularised structures and association with drug delivery to the CNS, respectively.
- Less than 10 µm pose the greatest risk for off-target delivery, as they are likely to reach the lungs.
Cascade impaction is the method used to quantify the mass of the drug in this last category; the fines fraction that presents a safety risk because of the likelihood of pulmonary deposition. Engineering products to minimise this fraction may be crucial from a safety perspective or indeed to demonstrate bioequivalence.
2. Why cascade impaction is the most relevant technique for off-target drug delivery
The value of cascade impaction stems from its ability to precisely measure the aerodynamic particle size distribution (APSD) of the active pharmaceutical ingredient, as opposed to the whole formulation, and because it provides good resolution in the size range of interest.
Aerodynamic measurements of particle size are intuitively appropriate when it comes to investigating the movement of particles in air. For suspension nasal drug products and nasal powders, drug-specific measurement is vital since the drug is not necessarily uniformly distributed within the formulation. The measurement range of cascade impaction is perfectly matched to regulatory requirements to assess the sub-10 µm fraction for nasal drug products.
3. How cascade impaction is implemented for nasal drug products
The image below shows two cascade impactor test set-ups for nasal drug product testing. One of the most noticeable shared features is the glass expansion chamber. Actuation into this chamber allows atomisation and dispersion of the dose prior to sampling in the impactor, with 1 L, 2 L and 5 L volumes. The aim is to optimise the method for a ‘worst-case’ scenario regarding pulmonary deposition by choosing an expansion chamber that maximises aerosolisation of the emitted dose entering the impactor.

The set-up on the left shows an abbreviated cascade impactor stack that might be used for nasal sprays. Many commonly used nasal drug devices generate minimal fines. The regulatory requirement is therefore to simply determine the total drug quantity beneath the first impactor stage. Using an abbreviated stack such as the Fast Screening Andersen at 28.3 L/min easily meets this requirement by producing > 5.0 µm, 1 – 5.0 µm, and sub-1.0 µm fractions. These fractions correlate with deposition in the upper respiratory tract, deep lungs, and oral exhalation respectively, offering a robust evaluation of off-target delivery. Glass expansion chambers of 2 or 5 L are usually the preference for nasal sprays.
The set-up on the right is for full-resolution cascade impaction, as required for nasal aerosols, using a Next Generation Impactor. Propellant-driven devices disperse doses more energetically, leading to increased fines, necessitating a more thorough characterisation of the sub-10 µm dose, relative to nasal sprays. Additionally, nasal aerosols typically require a smaller expansion volume; hence, testing specifies a 1 L expansion chamber.
For nasal powders, full resolution cascade impaction requires testing to be conducted under comparable conditions to those used for dry powder inhalers. There is no requirement for a glass expansion chamber; the standard Ph. Eur./USP induction port is used to interface the device and impactor instead.
Looking ahead
The growing interest in intranasal drug delivery underscores an increasing need for scrutiny of test methods and potential for improvement. Pioneers in this area are for example:
- Using shake, fire, and flow control automation platforms for drug product actuation in line with USP <601> which recommends ‘the use of a mechanical means of actuating the metering system or pump assembly to deliver doses for collection’ to ensure reproducibility.
- Transitioning from a glass expansion chamber and/or standard induction ports to anatomically accurate nasal inlets to produce a more clinically representative sample for cascade impactor measurements.
- Experimenting with more realistic breath profile flow rates to help understand how patient practice influences nasal drug product performance.
For further insights into nasal drug product testing, I’d recommend this Q&A with Matthew Fenn, Copley’s Head of Business Development. Also, look out for our next blog which will focus on the differences between laser diffraction and cascade impaction and why they are complementary and not alternative techniques for nasal drug products.








