Contact Us
Contact Us

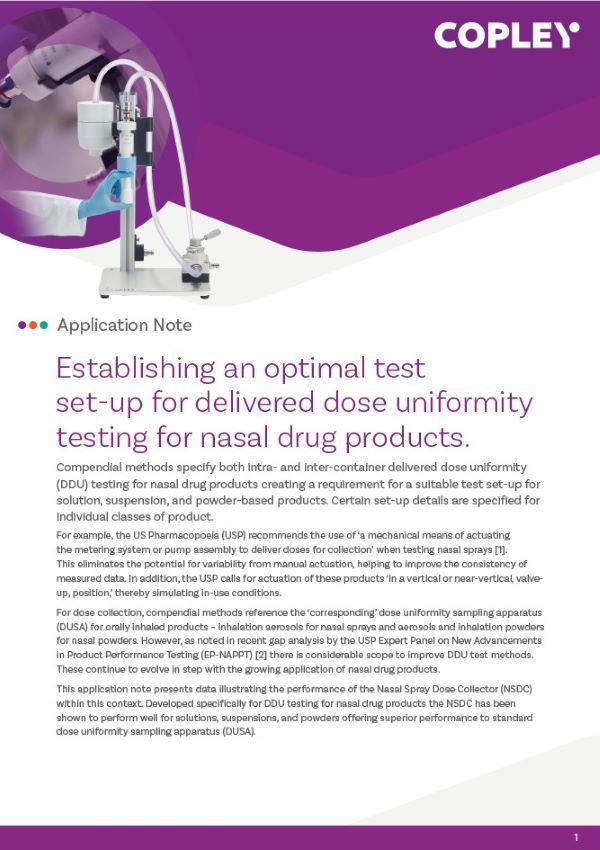
BRS 100i Breathing Simulator
BRS 100i is specifically designed for generating neonatal, infant, child and adult breathing patterns required for dose uniformity testing of nebulisers in accordance with ISO 27427:2013. It can also be used to generate the profiles required in USP <1602> for testing ‘Spacers and Valve Holding Chambers used with Inhalation Aerosols’.
Ph. Eur. 2.9.44, USP <1601>, <1602> and ISO 27427:2013 compliant
Intuitive, touchscreen user interface
Extensive data output options
|
BRS 100i Technical Specifications
|
|
|---|---|
|
Volume (manually adjust):
|
0 to 800 mL
|
|
Frequency:
|
12 – 40 bpm
|
|
I:E ratio:
|
1:1, 1:2 or 1:3
|
|
Waveforms:
|
Sinusoidal
|
|
Patient profile suitability:
|
Neonate/Infant, Child, Adult
|
|
Profile:
|
Inhalation and Exhalation
|
|
Start:
|
Select start on inhalation or exhalation stroke
|
|
User interface:
|
Touchscreen
|
|
Uses:
|
Testing Nebulisers (Ph. Eur. 2.9.44 and USP <1601> Testing MDIs with Spacers/VHCs (USP <1602> Improving IVIVCs for MDIs with Spacers/VHCs and nebulisers: With Filter Holder and Adapter (Dose Uniformity) With Impactor and Mixing Inlet (APSD)
|
Top Tip
The BRS 100i breathing simulator can also be used in place of a standard vacuum pump with a cascade impactor such as the NGI or ACI and a Mixing Inlet to form a simple and inexpensive system for APSD studies utilising more representative patient profiles for MDIs with and without a spacer/VHC.
Related Systems


Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out More