Contact Us
Contact Us
Please select an option:
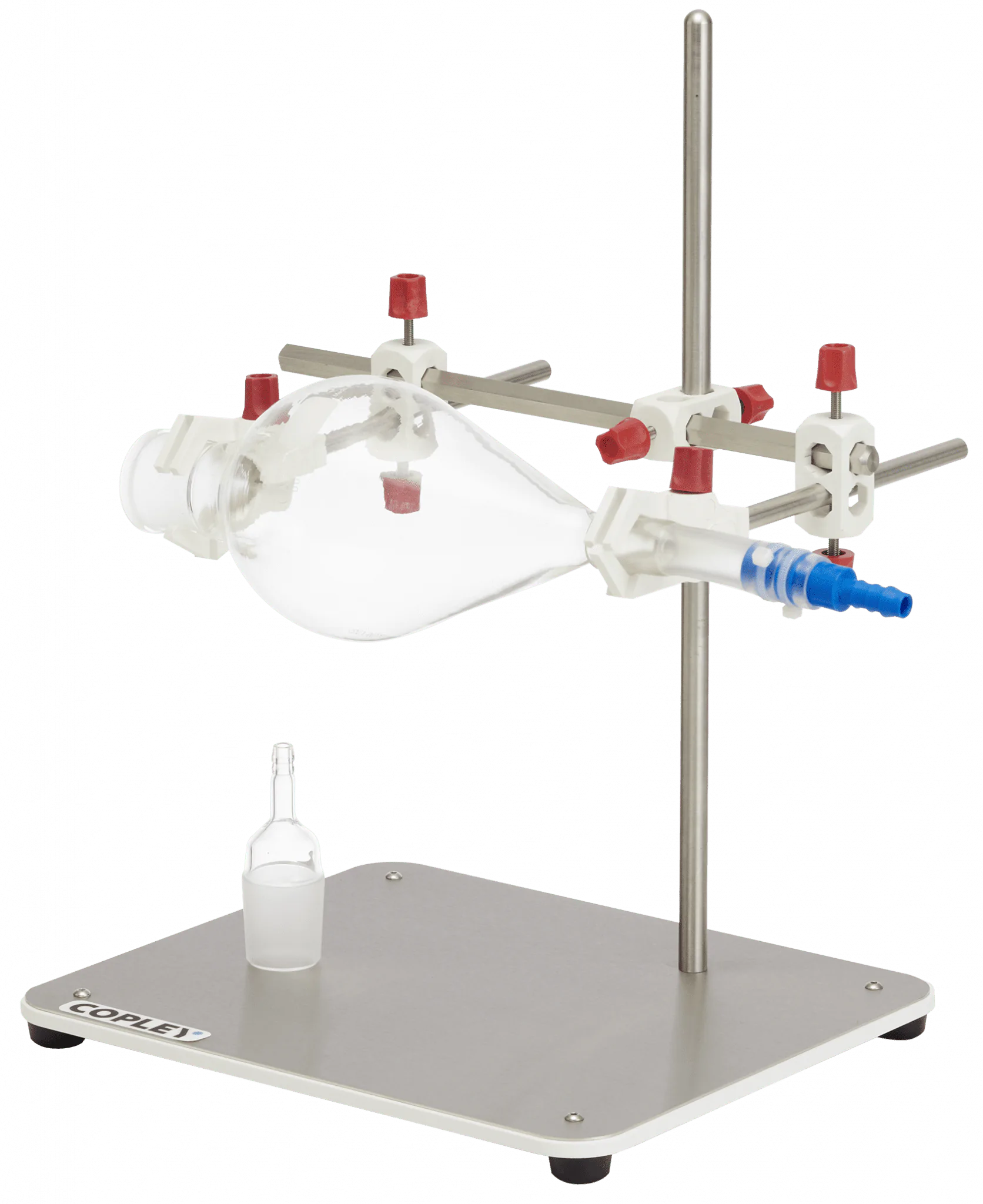
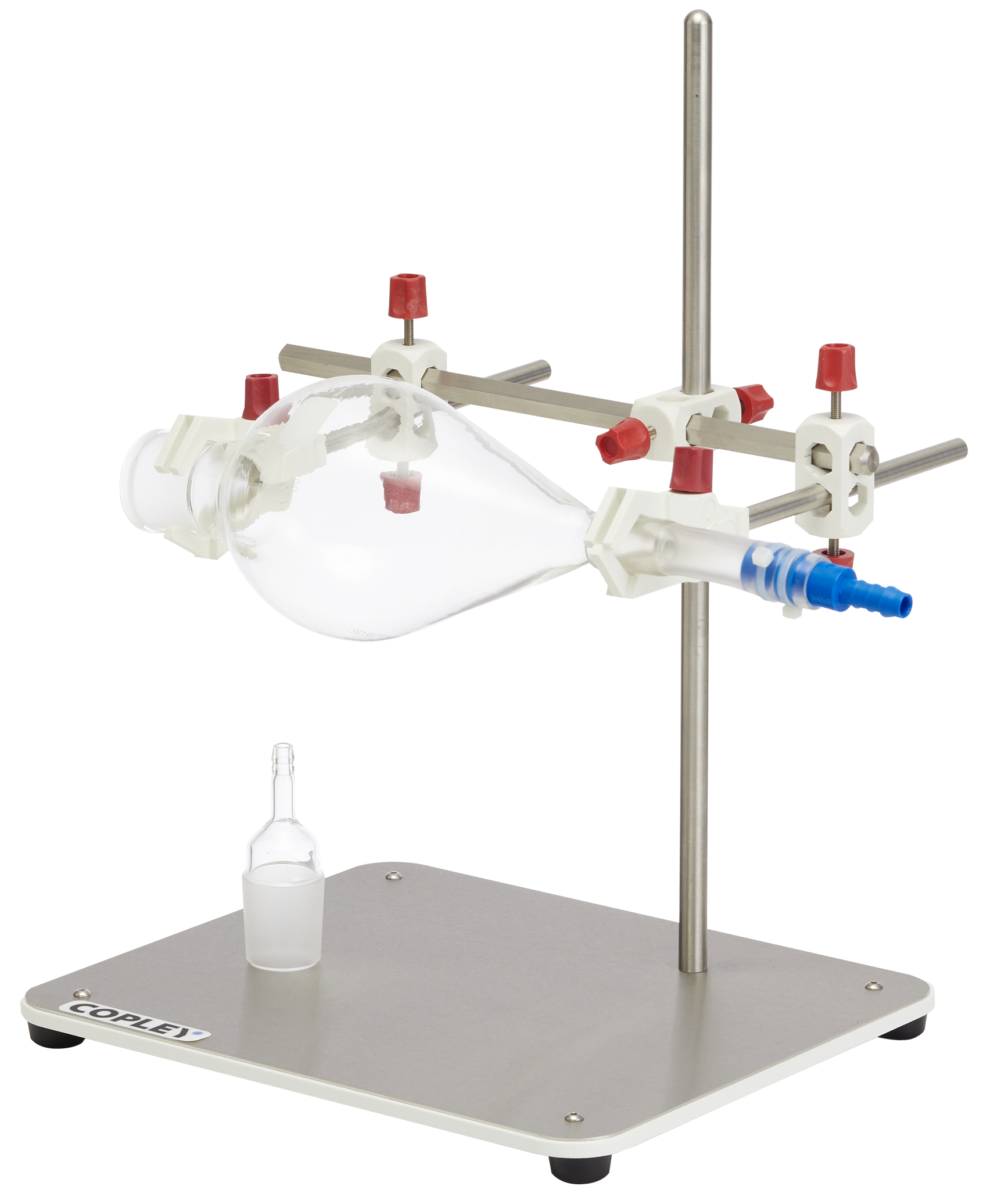
The monograph for Fluticasone Propionate Inhalation Aerosol specified that DDU measurements be conducted using a specific glass sampling apparatus.
Products featured here: |
|
Please select an option:

The monograph specified APSD measurement to be conducted using a standard Andersen Cascade Impactor (ACI) equipped with a specially modified induction port common to both aerosols and powders. Additionally, a specially modified inlet cone and preseparator are utilised for aerosols and powders, respectively.
As per the monographs, the 28.3 L/min version of the ACI (Stages 0 – 7 plus filter stage) should be employed to measure Aerodynamic Particle Size Distribution (ASPD) for both aerosols and powders, even though the powder method specifies testing at 60 L/min.
Products featured here: |
|
Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out More