Please select an option:
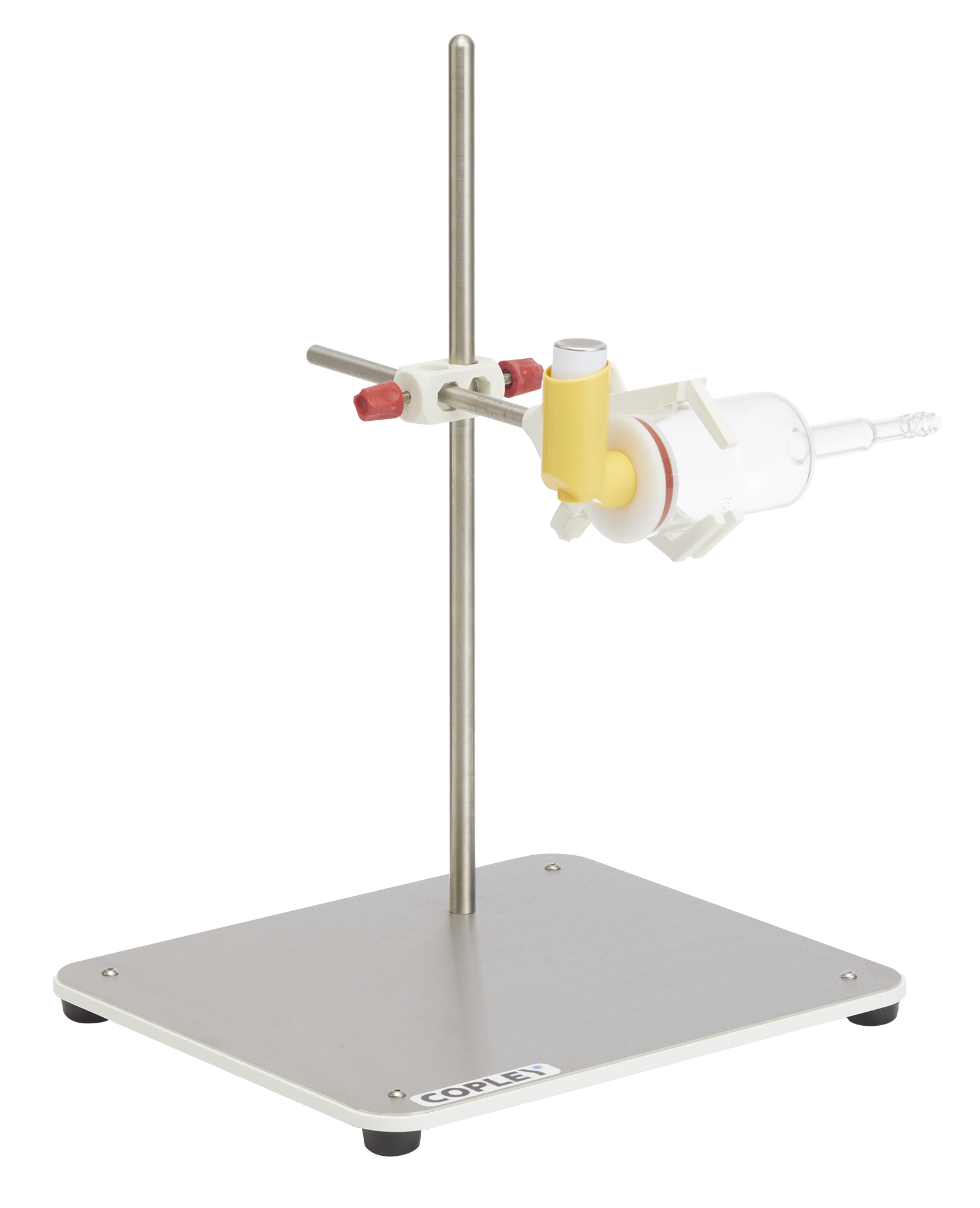
The draft monograph for Albuterol Inhalation Aerosol specified a special glass Sample Collection Apparatus to be used for DDU. The apparatus used a solid plastic firing adapter, instead of a mouthpiece adapter, to accept an inhaler with a circular mouthpiece of corresponding dimensions.
Products featured here:
|
|
Please select an option:

The draft monograph for Albuterol Inhalation Aerosol specified measurement using standard ACI equipped with a specially modified induction port. A special inlet sleeve is available that slips onto the induction port inlet, enabling the induction port to be used with regular mouthpiece adapters used on USP/NGI induction ports.
Products featured here: |
|
Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out More

