Our highlights from a key fixture in the inhaled drug delivery conference calendar
Edinburgh dressed in Christmas finery, expert speakers from every corner of the globe, and networking opportunities par excellence: it can only be the Drug Delivery to the Lungs conference. A lynchpin of the orally inhaled and nasal drug product (OINDP) calendar, DDL 2023 saw the conference well and truly return to its former glory. The whole Copley team had an excellent three days, here’s why:
We were inspired: by meeting Carrie Vaillencourt, a medical advisor at UPC Cambridge Ltd, currently training for the 2024 London Marathon. A little over a year ago Carrie was fully paralysed by a stroke and thought she would never recover. Determined to fight, she ‘walked’ a short distance only a few weeks after the stroke, taking 6 minutes to go just a few metres. One year on her progress is astounding. Carrie, it was great to see you at DDL and we’ll be rooting for you next year.
We were proud: to see our products referenced by so many researchers, in presentations and posters. Too many to mention but examples we’d like to highlight include the Annual DDL Lecture (see below), and a podium talk and poster by Patrica Henriques from Hovione and Andrew Martin from the University of Alberta, respectively, both of which showcased the Alberta Idealised Nasal Inlet for nasal deposition studies. Seeing our solutions generate the data that’s underpinning progress in key areas, including intranasal drug delivery is a heartening reminder of the contribution we make.
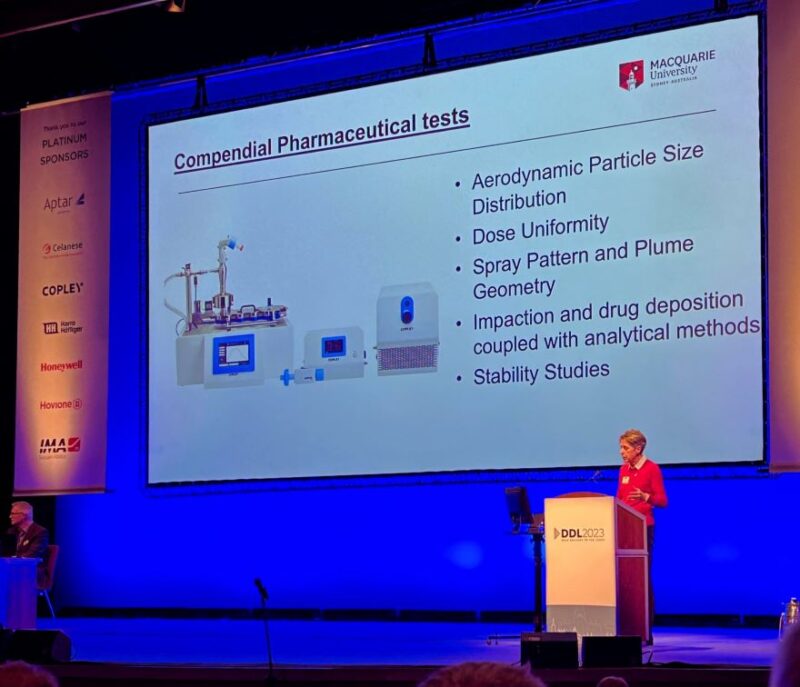
Daniela Traini delivering the DDL Annual Lecture
We were motivated: by the focus on emerging challenges for in vitro testing notably within the context of reducing reliance on other complex forms of testing. Professor Traini’s lecture ‘Of mice and men: Correlation and clinical relevance of animal models and non-clinical tests,’ for example, explored how, thanks to the FDA Modernization Act (2022) it is now possible to proceed to human clinical trials via non-clinical studies alone. It was a thought-provoking session that highlighted the use of tools such as cells and lab-grown tissues, organs on a chip, and computer models to reduce reliance on animal models, something I think we can all agree would be a great step forward.
Achieving better in vitro-in vivo correlations (IVIVCs) than the compendial methods allow was, more generally, a common topic of conversation. This has been an area of focus for several years now, so we already have a comprehensive range of products that can help those that are interested.

We were delighted: with the response to Vertus® III+ and DecaVertus® III, our new fully automated shake, fire, flow control and shot weight measurement platforms for nasal spray and metered dose inhaler (MDI) testing, which debuted at DDL. Existing customers were impressed by the new features, while those new to the Vertus concept were struck by its potential. One visitor from a leading inhalation contract development and manufacturing organisation was so impressed on Day 1, that they returned the very next day with a contact from another product development company for a further demo. We can’t ask for a better recommendation than that.
In addition, a poster talk delivered by our own Ben Bradley was also very well-received. ‘Matching DUSA and Impactor Rise-Time Profiles with a Volume and Resistance Compensator’ is a great watch for anyone looking to improve their inhaler testing practice, particularly for dry powder inhalers (DPIs).
We were curious: to find out more about the possible impacts of data science, AI, and developments in the modelling and simulation of OINDPs. Momentum appears to be gathering across these fields with recent advances in modelling particularly exciting to see. However, the results are complex, and the extensive validation required remains very much a work in progress.
We were ready: for a good catch-up on all the dominant industry themes including MDI reformulation with low Global Warming Potential (low GWP) propellants, inhaled biologics, and an increased focus on intranasal drug delivery. There were lots of valuable talks and posters in these areas, relating to the persistent problem of patient adherence, which continues to have a significant impact on patient outcomes and healthcare budgets. Perhaps digital solutions and AI will ultimately help to crack this thorny issue?
We really enjoyed: being back in Edinburgh, seeing familiar faces missed in recent years, and being part of this leading conference. Stimulating talks, lots of excellent posters, a busy exhibition, great social events and of course, bagpipes. What’s not to love?









