Contact Us
Contact Us
Key Features: Vertus® III
Ph. Eur., EMA, USP, FDA, ChP and NMPA compliant
21 CFR part 11 compliant
Precise control over all test parameters
Suitable for a wide range of devices

Compatible with all standard collection devices
In situ impactor leak testing capability

Wide range of data output options
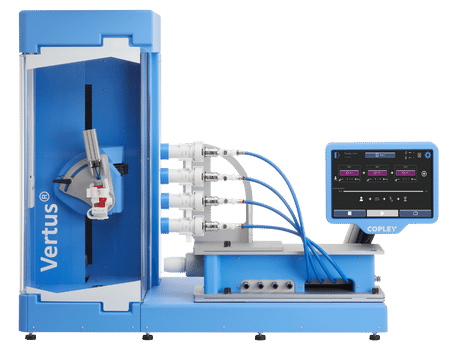
Precision-controlled, highly repeatable MDI, nasal spray and nasal aerosol testing
Vertus® III offers absolute control over a wide range of specific method parameters, including:
- Shaking profile (including speed, angle and duration)
- Time between shake and fire
- Firing profile (including force, pause, fire down, rise and release time)
- Air flow through system
The Vertus® III Range – Automated Shake and Fire Systems for MDIs and Nasal Sprays


NEW: Priming & Waste Module
Included as standard, the new Priming & Waste Module integrates firing-to-waste into automated test methods, enabling compendial entire contents testing with minimal manual input.
Each interface plate can be placed directly on top of the Priming & Waste Module. Vertus III and Vertus III+ can switch automatically between priming and test levels, firing-to-waste or to dose collection as required, without operator intervention. This enables highly efficient testing procedures, most notably to meet through-life test requirements for DDU and APSD.


NEW: Exhaust Port
The Exhaust Port supports the efficient extraction of flammable propellants or high potency drugs where additional safety measures are required, for example, as part of pMDI reformulation studies.
Vertus® III: Interface Plates
All Vertus® III interface plates are interchangeable, meaning analysts can easily swap between collection devices. No tools are required, simply lift and switch!
Over 40 collection device combinations are available, a selection of which are shown below. For a full list of all available interface plates, contact us at sales@copleyscientific.co.uk.


DUSA Stack with Priming & Waste Module
DUSA Interface Plate for Nasal Sprays
Next Generation Impactor NGI with Priming & Waste Module
Andersen Cascade Impactor ACI with Glass Expansion Chamber
Andersen Cascade Impactor ACI with Adult Alberta Idealised Throat and Priming & Waste Module
Next Generation Impactor NGI with Alberta Idealised Nasal Inlet AINI
Spray Force Tester SFT
Thin Layer Chromatography TLC Plate for Spray Pattern


Method Transfer: DecaVertus® III
Vertus® III is fully compatible with DecaVertus® III. Therefore methods can be easily transferred between systems as the product proceeds to commercialisation, with DecaVertus used in production to alleviate the burden of MDI through-life testing.
Find Out More: DecaVertus® IIIVertus III®: Technical Specifications | |
Device compatibility: | Metered Dose Inhalers (MDIs), Nasal Sprays, Nasal Aerosols |
Shot weight measurement: | No |
Static Eliminator included: | No |
Dimensions (w x d x h): | 1020 x 510 x 920 mm |
Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out More