Contact Us
Contact Us

Like nasal sprays, nasal aerosols also typically produce droplets in the range 20-200 microns, which is outside the effective range of cascade impactors. However, most aerosols typically deliver a proportion (typically <5%) of fine droplets in the <10 micron range, which must be quantified.
Used together with a cascade impactor such as the Andersen Cascade Impactor (ACI) and a high volume expansion chamber, such as the Glass Expansion Chamber, the Vertus® III/Vertus® III+ automated shake, fire and flow control systems can be used to meet the need for highly consistent nasal aerosol actuation as recommended by USP <601>.
Shot weight measurement
The Vertus® III+ model includes an integrated analytical balance for automated nasal spray shot weight measurement.


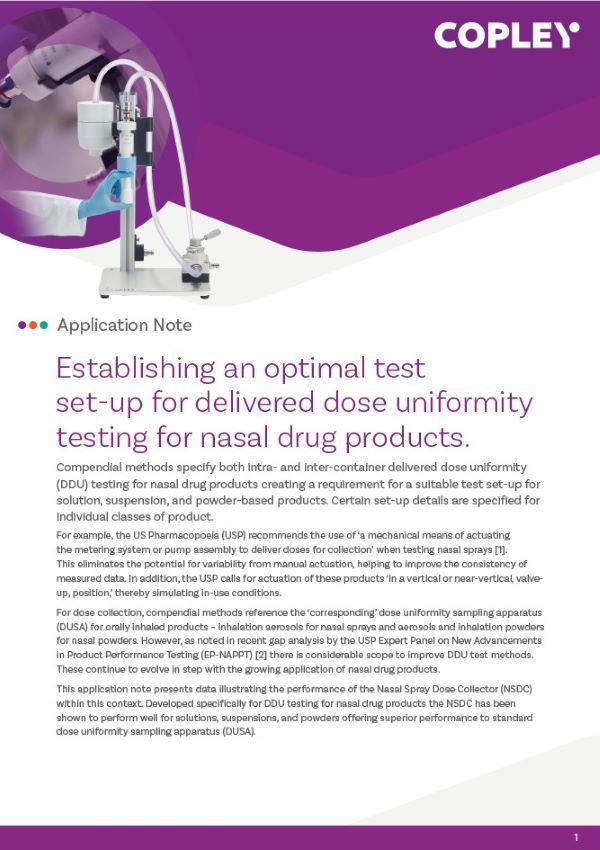
Nasal Aerosols: Semi-Automated APSD Measurement
The Vertus® III automated shake, fire and flow range is made up of integrated turn-key solutions for precise, controlled and reproducible nasal spray testing. Compatible with most nasal aerosols, the Vertus III or Vertus III+ offers analysts complete control over:
- The speed, angle and duration of shaking, ahead of actuation
- Firing force and the speed of application and release of that force
- The time delay between the end of shaking and device actuation
How Does a Cascade Impactor Work?


Manual Nasal Aerosol Sampling
In addition to the Vertus® automated shake, fire and flow systems, we offer a full suite of products to support manual testing, including flow meters, flow controllers and vacuum pumps.
View the manual systemRelated Applications
We also offer a range of equipment for additional nasal aerosol testing application support:
Semi-Automation Tools
Related Services



Training
We offer a range of training courses, presentations and seminars covering a wide range of topics.
Find Out More


Servicing
A comprehensive range of both in-house and on-site product servicing options are available
Find Out More


Support
Our team of experienced technicians and engineers are on hand to help and advise
Find Out More